Last Updated: September 23, 2025 | Reading Time: 18 minutes
🔬 Quick Answer
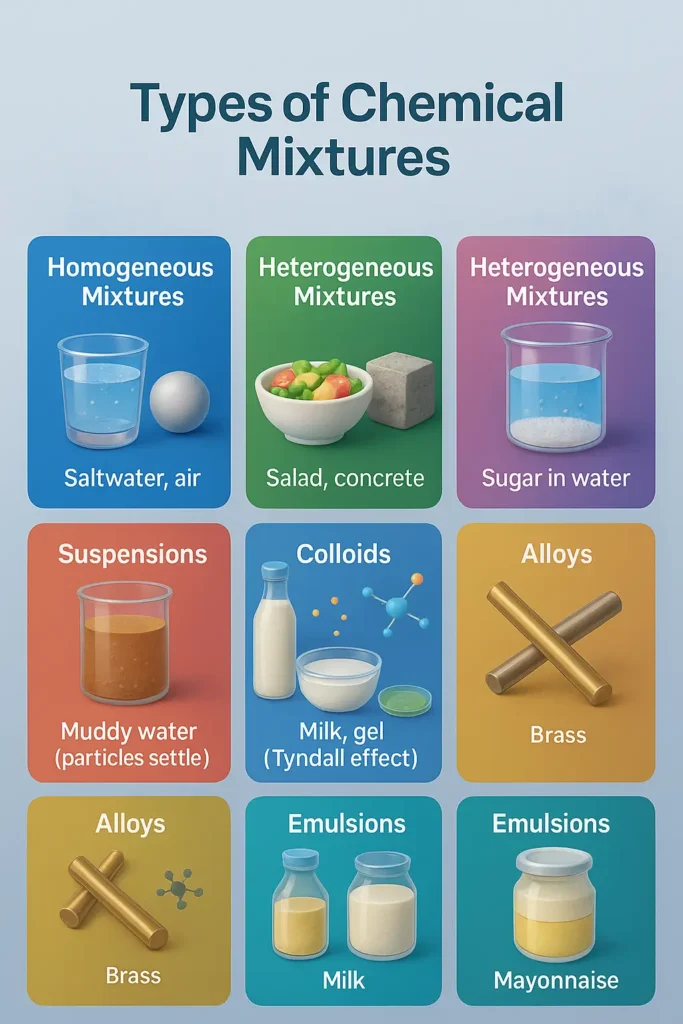
What are the 7 types of chemical mixtures? Chemical mixtures are physical combinations of substances that retain individual properties. The 7 main types are: Solutions (salt water), Colloids (milk), Suspensions (muddy water), Emulsions (mayonnaise), Foams (whipped cream), Gels (jelly), and Aerosols (fog). Each type has distinct particle sizes, stability, and separation methods crucial for chemistry students and professionals.
🎯 Key Takeaways Box
- Homogeneous mixtures have uniform composition (solutions)
- Heterogeneous mixtures have visible phases (all others)
- Particle size determines mixture type and properties
- Separation methods depend on mixture classification
- Industrial applications span pharmaceuticals to manufacturing
What Are Chemical Mixtures?
A chemical mixture consists of two or more substances physically combined without forming chemical bonds. This fundamental distinction from compounds means each component maintains its original molecular identity and properties, allowing separation through physical methods.
Consider your morning coffee – it perfectly demonstrates mixture principles where coffee compounds dissolve in water. Each substance retains its individual characteristics, explaining why you can separate them through evaporation or distillation processes.
🔬 Scientific Definition & Properties
Physical Combination: Components mix without creating new chemical bonds, preserving their molecular identities and original properties throughout the combination process.
Variable Composition: Unlike compounds with fixed stoichiometric ratios, mixtures can contain varying amounts of each component while maintaining their fundamental classification and behavior patterns.
Separable Components: Physical separation methods including filtration, distillation, chromatography, or magnetic separation can isolate individual components without chemical decomposition.
Retained Properties: Each component maintains its original chemical and physical characteristics, though these may be masked or modified by interactions with other components present.
📊 Mixtures vs Compounds: Critical Comparison
| Aspect | Chemical Mixtures | Chemical Compounds |
|---|---|---|
| Formation | Physical combination | Chemical bonding (ionic/covalent) |
| Composition | Variable ratios | Fixed ratios (stoichiometric) |
| Properties | Components retain individual properties | New properties formed |
| Separation | Physical methods | Chemical decomposition required |
| Energy Change | Usually no energy change | Energy absorbed or released |
| Examples | Salt water, air, soil | Water (H₂O), salt (NaCl), sugar (C₁₂H₂₂O₁₁) |
Homogeneous vs Heterogeneous Mixtures
Understanding mixture classification helps predict behavior, select appropriate analytical methods, and determine optimal separation techniques for laboratory and industrial applications.
✨ Homogeneous Mixtures (Single Phase Systems)
Homogeneous mixtures display uniform composition throughout, creating a single visible phase where components achieve complete molecular or ionic integration.
🔍 Key Characteristics:
- Uniform composition regardless of sampling location
- Single visible phase with no distinguishable boundaries
- Complete molecular or ionic level mixing
- Consistent physical and chemical properties throughout
- Components cannot be distinguished visually or with basic microscopy
🌟 Real-World Examples:
- Salt water: Sodium chloride completely dissolved in water at ionic level
- Air: Nitrogen, oxygen, and trace gases uniformly distributed
- Brass alloy: Copper and zinc atoms uniformly distributed in crystal lattice
- Vinegar: Acetic acid molecularly dispersed in water solution
💡 Pro Tip: Homogeneous doesn’t mean pure – brass appears uniform but contains multiple metals.
🔄 Heterogeneous Mixtures (Multi-Phase Systems)
Heterogeneous mixtures contain multiple visible phases with non-uniform composition and distinguishable boundaries between different components or regions.
🔍 Key Features:
- Non-uniform composition varying significantly by sampling location
- Multiple visible phases or distinct regions with clear boundaries
- Observable interfaces between different components (often visible to naked eye)
- Properties vary throughout different regions of the mixture
- Components frequently distinguishable through visual inspection
🌟 Common Examples:
- Oil and water: Clear density-based layer separation with visible interface
- Sand and iron filings: Visually distinct components with different magnetic properties
- Granite rock: Visible crystals of quartz, feldspar, and mica minerals
- Chocolate chip cookies: Clear distinction between dough matrix and chocolate pieces
The 7 Essential Types of Chemical Mixtures
1. 💧 Solutions (Homogeneous Systems)
Solutions represent the most fundamental homogeneous mixtures where solutes completely dissolve in solvents, creating stable, uniform systems with true molecular-level mixing and optical transparency.
🔬 Key Properties:
- Complete solute dissolution at molecular or ionic level
- Uniform composition throughout entire volume
- Light transparency with no scattering effects
- Long-term stability without settling or phase separation
- Particle size: <1 nanometer (molecular/ionic scale)
⚗️ Formation Process: When table salt dissolves in water, sodium and chloride ions separate completely and become surrounded by water molecules through hydration. This ionic dispersion creates a homogeneous solution where every sample contains identical ion concentrations, demonstrating true molecular-level mixing.
🏭 Industrial Applications:
- Pharmaceutical preparations: IV solutions, liquid medications, injectable drugs
- Electroplating solutions: Metal coating processes in automotive and electronics
- Chemical processing: Synthesis reactions, catalytic processes, purification
- Food and beverage: Soft drinks, alcoholic beverages, flavoring systems
📊 Types of Solutions by Physical State:
| Solution Type | Solute State | Solvent State | Examples | Applications |
|---|---|---|---|---|
| Liquid Solutions | Solid/Liquid/Gas | Liquid | Sugar water, alcohol, carbonated water | Pharmaceuticals, beverages |
| Gaseous Solutions | Gas | Gas | Air, natural gas | Industrial processes, breathing systems |
| Solid Solutions | Solid | Solid | Steel, brass, bronze | Metallurgy, construction materials |
2. 🥛 Colloids (Intermediate Dispersions)
Colloids contain intermediate-sized particles (1-1000 nanometers) that remain permanently suspended, creating unique optical and mechanical properties between true solutions and coarse suspensions.
🔬 Distinctive Features:
- Tyndall effect: Light beam becomes visible when passed through due to particle scattering
- Brownian motion: Random particle movement caused by molecular collisions
- Stable dispersion: Particles remain suspended indefinitely under normal conditions
- Optical properties: Often opaque, translucent, or opalescent appearance
- Intermediate particle size: Large enough to scatter light, small enough to resist settling
📊 Classification of Colloidal Systems:
| Colloid Type | Dispersed Phase | Continuous Phase | Common Examples | Key Properties |
|---|---|---|---|---|
| Sol | Solid | Liquid | Paint, ink, blood | Fluid behavior, settles under extreme conditions |
| Gel | Liquid | Solid Network | Gelatin, hair gel, hydrogels | Semi-solid, elastic, high water content |
| Emulsion | Liquid | Liquid | Milk, mayonnaise, butter | Requires emulsifier, can be O/W or W/O |
| Foam | Gas | Liquid | Whipped cream, soap suds | Low density, unstable, surface tension effects |
| Aerosol | Solid/Liquid | Gas | Smoke, fog, spray paint | Atmospheric suspension, light scattering |
🏥 Advanced Medical Applications:
- Targeted drug delivery: Nanoparticle carriers for cancer treatment
- Contact lenses: Biocompatible hydrogel materials for vision correction
- Wound care systems: Moisture-retaining hydrogel dressings for healing
- Blood substitutes: Colloidal plasma expanders for emergency medicine
- Diagnostic imaging: Contrast agents for MRI and CT scans
💊 Clinical Reference: FDA Guidance on Colloidal Drug Products
3. 🌊 Suspensions (Coarse Dispersions)
Suspensions contain the largest particles (>1000 nanometers) among mixture types, with particles large enough for visual identification and eventual gravity-induced settling.
🔍 Identifying Characteristics:
- Large, visible particles observable with naked eye or basic magnification
- Settling behavior: Particles separate over time due to gravitational effects
- Turbid appearance: Cloudy or opaque due to light scattering by large particles
- Easy filtration: Simple filter paper can separate components
- Temporary stability: Requires continuous agitation to maintain dispersion
🌟 Everyday Examples:
- Muddy water: Soil particles temporarily mixed with water, clear settling layers
- Oil-vinegar dressing: Large oil droplets that quickly separate and float
- Calamine lotion: Zinc oxide particles dispersed in water-based medium
- Chalk dust in air: Calcium carbonate particles temporarily airborne
💊 Pharmaceutical Applications: Many medications are formulated as suspensions when active ingredients exhibit poor water solubility. These formulations require “shake well before use” instructions because active particles settle during storage, potentially affecting dosage accuracy.
Examples:
- Antibiotic suspensions: Amoxicillin for pediatric use
- Antacids: Aluminum hydroxide/magnesium hydroxide combinations
- Topical treatments: Calamine lotion for skin conditions
4. 🧈 Emulsions (Liquid-Liquid Colloids)
Emulsions represent specialized colloidal systems where two immiscible liquids mix with emulsifying agents, creating stable dispersions that would otherwise rapidly separate.
⚗️ Formation Requirements:
- Two immiscible liquids: Typically oil and water phases with different polarities
- Emulsifying agent: Surfactant with both hydrophilic and lipophilic properties
- Mechanical energy: Mixing, homogenization, or sonication for droplet formation
- Optimal conditions: Controlled temperature, pH, and ionic strength
📊 Emulsion Classification:
| Type | Structure | Examples | Characteristics | Applications |
|---|---|---|---|---|
| Oil-in-Water (O/W) | Oil droplets in water | Milk, mayonnaise, face creams | Easier cleanup, lighter feel | Cosmetics, food products |
| Water-in-Oil (W/O) | Water droplets in oil | Butter, margarine, cold cream | Occlusive properties, richer feel | Protective creams, spreads |
| Multiple Emulsions | W/O/W or O/W/O | Controlled-release formulations | Complex structure, sustained release | Advanced pharmaceuticals |
🔬 Stability Factors:
- Droplet size: Smaller droplets create more stable emulsions
- Emulsifier concentration: Higher concentrations improve stability
- Temperature effects: Heat can break or promote emulsification
- pH conditions: Affects emulsifier effectiveness
- Ionic strength: Salt content influences stability
🏭 Industrial Applications:
- Cosmetics industry: Creams, lotions, foundations, moisturizers
- Food processing: Sauces, dressings, spreads, ice cream
- Pharmaceutical formulations: Topical creams, injectable emulsions
- Paint and coatings: Latex paints, protective coatings, architectural finishes
5. 🫧 Foams (Gas-Liquid Dispersions)
Foams consist of gas bubbles dispersed in liquid or solid matrices, creating materials with exceptional properties including low density, thermal insulation, and unique mechanical characteristics.
🔬 Fundamental Properties:
- Low density: High gas content reduces overall material weight
- Thermal insulation: Trapped gas pockets minimize heat transfer
- Variable stability: Ranges from seconds (soap bubbles) to permanent (styrofoam)
- Elastic behavior: Ability to compress and recover shape
- Surface tension effects: Determine bubble size, shape, and stability
📊 Foam Categories and Applications:
| Category | Stability | Examples | Applications | Key Features |
|---|---|---|---|---|
| Temporary Foams | Seconds to hours | Soap bubbles, beer foam, whipped cream | Food, cleaning, entertainment | Short-lived, easily broken |
| Semi-permanent | Hours to days | Shaving cream, fire-fighting foam | Personal care, safety | Controlled breakdown |
| Permanent Foams | Years to indefinite | Styrofoam, foam rubber, concrete | Construction, packaging | Structural integrity |
🏗️ Construction Applications:
- Foam concrete: Lightweight building material with excellent insulation
- Spray foam insulation: Expanding polyurethane for energy efficiency
- Structural foams: Load-bearing applications in aerospace and automotive
🔥 Safety Applications:
- Fire-fighting foams: Create barrier between fuel and oxygen
- Emergency response: Specialized foams for chemical spill containment
6. 🍮 Gels (Semi-Solid Networks)
Gels combine liquid phases trapped within three-dimensional solid networks, creating semi-solid materials with unique mechanical properties and high liquid content.
🏗️ Structural Characteristics:
- Three-dimensional network: Cross-linked polymer, protein, or crystalline structure
- High liquid content: Often exceeding 90% while maintaining solid behavior
- Elastic properties: Ability to deform and recover original shape
- Shape retention: Maintains structure despite high liquid content
- Selective permeability: Allows controlled transport of molecules
📊 Gel Classification by Structure:
| Gel Type | Network Structure | Liquid Phase | Examples | Applications |
|---|---|---|---|---|
| Hydrogels | Polymer networks | Water | Contact lenses, wound dressings | Medical, biomedical |
| Organogels | Organic networks | Organic solvents | Cosmetic gels, pharmaceutical gels | Personal care, drugs |
| Aerogels | Porous networks | Air | Silica aerogel, carbon aerogel | Insulation, aerospace |
🏥 Biomedical Applications:
- Drug delivery systems: Controlled-release hydrogel matrices for sustained medication
- Tissue engineering: Biodegradable scaffolds for cell growth and organ regeneration
- Surgical implants: Biocompatible gels for various medical procedures
- Contact lenses: Oxygen-permeable hydrogels for vision correction
- Wound care: Moisture-maintaining environments for optimal healing
🌱 Agricultural Applications:
- Water retention: Superabsorbent gels for drought-resistant farming
- Slow-release fertilizers: Nutrient-containing gels for sustained plant feeding
- Soil conditioning: Improving water retention in arid regions
7. 🌫️ Aerosols (Gas Phase Dispersions)
Aerosols contain solid or liquid particles suspended in gases (typically air), playing crucial roles in atmospheric processes, industrial applications, and medical treatments.
🔬 Fundamental Characteristics:
- Particle size range: 0.001-100 micrometers determining behavior and applications
- Gas phase suspension: Particles remain airborne for extended periods
- Light scattering effects: Cause visibility reduction and optical phenomena
- Variable settling rates: Depend on particle size, density, and air currents
- Atmospheric significance: Critical for weather, climate, and air quality
🌍 Natural Aerosol Systems:
| Type | Particle Source | Size Range | Environmental Impact | Examples |
|---|---|---|---|---|
| Clouds | Water vapor condensation | 1-100 μm | Weather patterns, precipitation | Cumulus, stratus, cirrus |
| Fog | Ground-level condensation | 1-40 μm | Visibility, transportation | Radiation fog, advection fog |
| Smoke | Combustion processes | 0.01-10 μm | Air quality, climate | Wildfire, volcanic emissions |
| Dust | Wind erosion, human activity | 0.1-100 μm | Health, atmospheric chemistry | Saharan dust, pollen |
💊 Medical Aerosol Applications:
- Respiratory drug delivery: Metered-dose inhalers for asthma, COPD treatment
- Nebulizer therapy: Liquid medication conversion to inhalable mist
- Particle size optimization: Targeting specific lung regions for maximum efficacy
- Pulmonary diagnostics: Aerosol-based lung function testing
🏭 Industrial Aerosol Applications:
- Spray coating: Automotive paints, protective finishes, decorative applications
- Agricultural spraying: Pesticide, fertilizer, and herbicide distribution
- Air pollution control: Electrostatic precipitators, baghouse filters
- Manufacturing processes: Powder coating, spray drying, material processing
🔗 Research Reference: EPA Aerosol Research
Recent Research Breakthroughs in Mixture Science
🧠 Neurotoxic Effects of Chemical Mixtures (2024)
Recent 2024 research has revealed that chemicals in complex mixtures show cumulative neurotoxic effects even when individual concentrations are below traditionally considered harmful thresholds. This breakthrough understanding has significant implications for environmental safety assessments and occupational health protection.
🔬 Key Research Findings:
- Additive toxicity: Mixture effects exceed individual component toxicity predictions
- Concentration synergy: Ratios found in human populations show unexpected additive effects
- Assessment limitations: Traditional single-chemical testing significantly underestimates real-world risks
- Regulatory implications: Need for mixture-based safety evaluation protocols
📊 Impact on Safety Standards:
- Environmental protection agencies reviewing mixture assessment protocols
- Occupational safety limits being reconsidered for chemical combinations
- Consumer product safety evaluations incorporating mixture effects
🤖 AI-Driven Environmental Mixture Analysis (2024-2025)
Artificial intelligence applications are providing critical insights into how complex chemical mixtures in aquatic systems affect marine and freshwater ecosystems, representing a major advancement in environmental monitoring and protection.
💡 Technological Innovations:
- Real-time analysis: Continuous mixture composition monitoring in water bodies
- Predictive modeling: Machine learning algorithms forecast ecological impacts
- Pattern recognition: AI identification of harmful mixture combinations
- Automated systems: Unmanned monitoring stations with AI-powered analysis
🌊 Environmental Applications:
- Water quality assessment: Comprehensive mixture toxicity evaluation
- Pollution source identification: Tracing contamination origins through mixture fingerprinting
- Ecosystem protection: Early warning systems for aquatic life threats
- Remediation guidance: AI-optimized treatment strategies for contaminated sites
🔬 Nanotechnology and Advanced Mixture Systems
Recent developments in nanotechnology are revolutionizing mixture applications across multiple industries, from targeted drug delivery to environmental remediation.
🧬 Nanoparticle Mixture Innovations:
- Smart drug delivery: pH-responsive nanoparticle carriers for targeted therapy
- Enhanced catalysis: Nanostructured catalysts with improved efficiency and selectivity
- Water purification: Advanced nanofiltration systems for contaminant removal
- Energy storage: Nanocomposite electrodes for battery and supercapacitor applications
🔬 Environmental Nanotechnology: Nanopore technology enables rapid identification of multiple targets within complex environmental mixtures with minimal sample preparation, representing a breakthrough for real-time environmental monitoring and assessment.
🌱 Biochar-Enhanced Mixture Systems (2025)
Modified biochar systems demonstrate enhanced adsorptive capacity for removing various pollutants from water, including heavy metals and organic compounds, advancing sustainable water treatment technologies.
♻️ Sustainable Applications:
- Water treatment: Enhanced removal of pharmaceutical residues and industrial chemicals
- Soil remediation: Biochar mixtures for contaminated land restoration
- Carbon sequestration: Long-term atmospheric CO₂ reduction strategies
- Agricultural benefits: Improved soil fertility and water retention
Identification Methods for Different Mixture Types
👁️ Visual Assessment Techniques
Step 1: Initial Macroscopic Observation
- Examine overall mixture uniformity and phase distribution
- Identify visible boundaries, interfaces, or phase separations
- Assess color consistency and transparency levels
- Note any settling, layering, or creaming phenomena
Step 2: Magnification Analysis
- Use hand lens (10x) or stereomicroscope for detailed structure examination
- Identify individual particle sizes and distribution patterns
- Locate interface boundaries between different phases
- Assess degree of component separation and interaction
🔍 Visual Identification Guide:
| Mixture Type | Visual Appearance | Key Indicators | Common Mistakes |
|---|---|---|---|
| Solution | Clear, transparent | No visible particles, uniform color | May confuse with clear colloids |
| Colloid | Translucent to opaque | Uniform but not transparent | Mistaking for solutions when clear |
| Suspension | Cloudy, particles visible | Obvious settling, layered appearance | Confusing fresh suspensions with colloids |
| Emulsion | Creamy, uniform but opaque | No visible separation when stable | Missing temporary separation |
🧪 Essential Laboratory Tests
🔦 Tyndall Effect Test (Gold Standard for Colloids):
- Procedure: Shine intense light beam (laser pointer/flashlight) through sample
- Solutions: No visible light scattering, beam invisible within mixture
- Colloids: Strong light scattering, beam clearly visible throughout sample
- Suspensions: Intense scattering with visible particles disrupting beam path
⏰ Settling Test (Time-Based Analysis):
- Protocol: Allow mixture to stand undisturbed for specified time periods (1 hour, 24 hours)
- Suspensions: Clear settling with distinct layers forming progressively
- Colloids: No observable settling or separation over extended periods
- Solutions: No visible changes in appearance or composition
🗂️ Filtration Test (Size-Based Separation):
- Method: Pass mixture through standard filter paper (pore size ~10-25 μm)
- Suspensions: Particles retained on filter, clear filtrate obtained
- Colloids and solutions: Pass through unchanged (particles too small for retention)
🔬 Advanced Identification Techniques
📏 Particle Size Analysis:
- Dynamic Light Scattering (DLS): Precise measurement of colloidal particles (1-1000 nm)
- Laser Diffraction: Suspension analysis for particles >0.5 μm
- Electron Microscopy: Direct visualization of nanoscale structures
- Sedimentation Analysis: Particle size distribution through settling rates
🌈 Spectroscopic Methods:
- UV-Vis Spectroscopy: Concentration determination and component identification
- Infrared (FTIR) Spectroscopy: Molecular structure analysis and functional group identification
- Nuclear Magnetic Resonance (NMR): Detailed molecular structure and dynamics
- Raman Spectroscopy: Non-destructive molecular identification
⚡ Advanced Characterization:
- Zeta Potential: Surface charge measurement for colloidal stability assessment
- Rheological Testing: Flow behavior analysis for complex mixtures
- Thermal Analysis: Phase transition and stability studies
- Chromatographic Separation: Component identification and quantification
📚 Laboratory Reference: ASTM Standards for Mixture Analysis
Separation Techniques by Mixture Type
💧 Solution Separation Methods
🌡️ Distillation (Temperature-Based Separation):
- Simple Distillation: Single-stage separation for components with large boiling point differences (>25°C)
- Fractional Distillation: Multi-stage separation using packed columns for close boiling points
- Vacuum Distillation: Reduced pressure techniques for heat-sensitive compounds
- Steam Distillation: Water-steam co-distillation for essential oils and aromatics
💨 Evaporation Techniques:
- Simple Evaporation: Solvent removal leaving solid solute residue
- Rotary Evaporation: Controlled, reduced-pressure solvent removal
- Freeze Drying: Sublimation-based gentle solvent removal for heat-sensitive materials
🔷 Crystallization Methods:
- Cooling Crystallization: Temperature reduction to decrease solubility
- Evaporative Crystallization: Concentration increase through solvent removal
- Anti-solvent Crystallization: Addition of miscible non-solvent for precipitation
🥛 Colloidal System Separation
⚡ Electrokinetic Techniques:
- Electrophoresis: Electric field-induced particle separation based on charge and size
- Electrocoagulation: Electrical destabilization of colloidal particles
- Electrodialysis: Ion-selective membrane separation using electric fields
🔬 Membrane-Based Separation:
- Ultrafiltration: Size-based separation using semi-permeable membranes (1-100 nm pores)
- Dialysis: Concentration gradient-driven separation of small molecules
- Reverse Osmosis: Pressure-driven separation for very small particles and dissolved substances
⚗️ Destabilization Methods:
- Chemical Coagulation: Addition of coagulants (aluminum sulfate, ferric chloride) to aggregate particles
- pH Adjustment: Altering surface charge to promote particle aggregation
- Thermal Treatment: Heat-induced coagulation for protein-based colloids
🌊 Suspension Separation Techniques
🗂️ Mechanical Separation:
- Gravity Filtration: Simple filter paper separation using gravitational force
- Vacuum Filtration: Accelerated filtration using reduced pressure
- Pressure Filtration: High-pressure systems for difficult-to-filter suspensions
- Cross-flow Filtration: Continuous filtration with reduced membrane fouling
🌀 Centrifugal Methods:
- Laboratory Centrifugation: High-speed spinning for density-based separation (up to 100,000 x g)
- Industrial Centrifuges: Large-scale continuous separation systems
- Ultracentrifugation: Extreme speeds for very fine particle separation
⬇️ Gravitational Techniques:
- Sedimentation: Natural settling in large tanks or basins
- Decantation: Careful liquid removal after settling
- Thickening: Concentration of suspended solids through controlled settling
🧈 Emulsion Breaking Methods
🌡️ Thermal Demulsification:
- Heat Treatment: Temperature increase to reduce emulsifier effectiveness
- Freeze-Thaw Cycles: Physical disruption of emulsion structure
- Thermal Gradient: Controlled temperature changes for selective separation
⚗️ Chemical Demulsification:
- Demulsifying Agents: Chemical additives that destabilize emulsion interfaces
- pH Modification: Altering chemical environment to break emulsions
- Solvent Addition: Introduction of solvents to selectively dissolve phases
⚡ Physical Methods:
- High-Speed Centrifugation: Overcoming emulsion stability through extreme force
- Electrical Demulsification: Electric fields to coalesce dispersed droplets
- Membrane Separation: Selective permeation of continuous phase
📊 Separation Method Selection Guide
| Mixture Type | Primary Methods | Equipment Needed | Time Required | Efficiency |
|---|---|---|---|---|
| Solutions | Distillation, evaporation, crystallization | Heating, cooling, vacuum systems | Hours to days | 95-99% |
| Colloids | Electrophoresis, ultrafiltration, coagulation | Specialized membranes, electric fields | Minutes to hours | 90-98% |
| Suspensions | Filtration, centrifugation, settling | Filters, centrifuges, settling tanks | Minutes to hours | 98-99.9% |
| Emulsions | Demulsification, centrifugation, heating | Chemical additives, heat, centrifuge | Minutes to hours | 85-95% |
🔬 Industrial Reference: Perry’s Chemical Engineers’ Handbook – Separation Processes
Industrial & Medical Applications
💊 Pharmaceutical Industry Applications
🎯 Advanced Drug Delivery Systems:
- Liposomal Formulations: Colloidal carriers for targeted cancer therapy with reduced side effects
- Sustained-Release Gels: Hydrogel matrices for controlled medication release over days to weeks
- Inhalation Aerosols: Precisely sized particles (1-5 μm) for optimal lung deposition
- Injectable Emulsions: Parenteral nutrition and lipophilic drug delivery systems
- Transdermal Gels: Skin-penetrating formulations for systemic drug delivery
🔬 Quality Control and Manufacturing:
- Solution Standardization: Precise concentration control for injectable medications
- Mixture Stability Testing: Shelf-life determination through accelerated aging studies
- Bioavailability Optimization: Formulation adjustments to improve drug absorption
- Contamination Detection: Advanced analytical techniques for purity verification
- Process Validation: Ensuring consistent mixture quality throughout production
📊 Regulatory Compliance:
- FDA Guidelines: Adherence to Current Good Manufacturing Practices (cGMP)
- ICH Standards: International harmonization of pharmaceutical mixture testing
- Pharmacopoeial Requirements: Meeting USP, EP, and JP specifications
🍽️ Food and Beverage Industry
🥘 Product Development and Processing:
- Emulsion-Based Products: Mayonnaise, salad dressings, ice cream, margarine
- Foam-Structured Foods: Bread texture, whipped toppings, aerated chocolates
- Gel-Based Systems: Desserts, confections, meat products, dairy alternatives
- Solution Applications: Beverages, syrups, flavor extracts, liquid seasonings
⚗️ Food Processing Technologies:
- Homogenization: Creating stable emulsions in dairy products (milk, cream)
- Extraction Processes: Separating oils, essences, and active compounds from natural sources
- Purification Systems: Removing contaminants while preserving nutritional value
- Concentration Techniques: Evaporation and membrane separation for juice production
- Crystallization: Sugar refining and salt purification processes
🔍 Quality Assessment Methods:
- Mixture Analysis: Component identification and quantification for labeling accuracy
- Stability Testing: Shelf-life determination for complex food mixtures
- Texture Analysis: Rheological testing of emulsions, gels, and foams
- Sensory Evaluation: Consumer acceptance testing for mixture-based products
🌍 Environmental Applications
💧 Water Treatment Technologies:
- Coagulation-Flocculation: Removing suspended particles using aluminum and iron salts
- Advanced Oxidation: Breaking down organic pollutants in complex mixtures
- Membrane Separation: Reverse osmosis and ultrafiltration for water purification
- Biological Treatment: Microbial degradation of organic mixture components
- Adsorption Systems: Activated carbon for trace contaminant removal
🌬️ Air Quality Management:
- Aerosol Control: Electrostatic precipitators and baghouse filters for particle removal
- Scrubbing Systems: Gas-liquid contact for removing soluble pollutants
- Catalytic Treatment: Converting harmful gas mixtures to benign products
- Biofilters: Microbial treatment of volatile organic compound mixtures
🏭 Industrial Waste Management:
- Separation and Recovery: Extracting valuable components from waste mixtures
- Treatment Optimization: Selecting appropriate methods for specific mixture types
- Resource Recovery: Converting waste mixtures into useful products
- Environmental Remediation: Cleaning contaminated soil and groundwater
🏭 Manufacturing Industries
🔧 Materials Science Applications:
- Composite Materials: Fiber-reinforced plastics with controlled mixture ratios
- Advanced Alloys: Precision-controlled metallic mixtures for aerospace applications
- Catalytic Systems: Optimized mixture formulations for chemical processes
- Coating Technologies: Complex mixture formulations for protective and decorative finishes
⚙️ Process Industries:
- Petroleum Refining: Fractional distillation of crude oil mixtures
- Chemical Manufacturing: Reaction mixture optimization for maximum yield
- Metallurgical Processing: Ore separation and metal purification
- Polymer Production: Controlled polymerization in solution and emulsion systems
Common Misconceptions About Chemical Mixtures
❌ Misconception 1: Homogeneous Equals Pure
🔍 The Reality: Homogeneous mixtures appear uniform but contain multiple components with distinct identities. Brass looks like a pure, shiny metal but actually consists of copper and zinc atoms uniformly distributed throughout the crystal structure.
💡 Why This Matters: This misconception can lead to incorrect assumptions about material properties, processing requirements, and safety considerations in industrial applications.
✅ Correct Understanding: Homogeneous describes distribution uniformity, not chemical purity. Even perfectly mixed systems can contain multiple components.
❌ Misconception 2: All Clear Liquids Are Solutions
🔍 The Reality: Some colloidal systems can appear completely clear or nearly transparent to casual observation. Certain protein solutions and polymer dispersions demonstrate colloidal behavior while maintaining optical clarity.
💡 Laboratory Implications: Assuming clarity indicates solution behavior can lead to inappropriate analytical methods and incorrect processing decisions.
✅ Correct Approach: Use the Tyndall effect test and other analytical methods to definitively classify clear mixtures.
❌ Misconception 3: Mixtures Lack Emergent Properties
🔍 The Reality: While individual components retain their fundamental properties, mixtures can exhibit emergent behaviors not present in pure components. Steel alloys demonstrate superior strength compared to pure iron, and pharmaceutical formulations show enhanced bioavailability through mixture effects.
💡 Industrial Significance: Understanding emergent properties enables the development of advanced materials and optimized formulations.
✅ Correct Perspective: Mixtures can have properties that differ significantly from those of individual components while still maintaining component identities.
❌ Misconception 4: Physical Separation Always Requires Complex Equipment
🔍 The Reality: Many separation processes use simple, everyday techniques. Coffee brewing demonstrates filtration, evaporation of salt water shows crystallization, and oil-water separation illustrates decantation.
💡 Educational Value: Understanding simple separation methods builds intuition for more complex industrial processes.
✅ Practical Approach: Start with simple separation principles before progressing to advanced techniques.
Interactive Elements
🧪 Mixture Identification Quiz
Test Your Knowledge: Identify the Mixture Type
- Milk – What type of mixture is milk?
- A) Solution B) Colloid C) Suspension D) Emulsion
- Answer: D – Emulsion (fat droplets in water with natural emulsifiers)
- Muddy Water After Rain – How would you classify this?
- A) Solution B) Colloid C) Suspension D) Gel
- Answer: C – Suspension (large soil particles that settle)
- Clear Gelatin Dessert – What mixture type does this represent?
- A) Solution B) Colloid-Gel C) Suspension D) Foam
- Answer: B – Colloid-Gel (protein network trapping water)
📊 Interactive Mixture Properties Comparison
| Property | Solutions | Colloids | Suspensions | Emulsions |
|---|---|---|---|---|
| Particle Size | <1 nm | 1-1000 nm | >1000 nm | 10-1000 nm |
| Tyndall Effect | ❌ No | ✅ Yes | ✅ Strong | ✅ Yes |
| Settling | ❌ Never | ❌ No | ✅ Yes | ⚠️ Eventually |
| Filtration | Passes through | Passes through | ✅ Retained | Passes through |
| Stability | Permanent | Permanent | Temporary | Variable |
🔬 Virtual Laboratory Exercises
Exercise 1: Tyndall Effect Demonstration
- Prepare three samples: salt water, milk, and muddy water
- Shine flashlight beam through each sample
- Record observations and classify each mixture type
- Explain the optical differences observed
Exercise 2: Separation Challenge
- Given: Sand, salt, and iron filing mixture
- Design separation procedure using available equipment
- Consider: magnetism, solubility, and filtration
- Execute separation and analyze results
FAQ
❓ What are the 4 main types of mixtures?
The four fundamental mixture categories are solutions (homogeneous, like salt water), colloids (intermediate particles, like milk), suspensions (large particles that settle, like muddy water), and emulsions (liquid-in-liquid systems, like mayonnaise). However, the complete classification includes seven specific types when considering foams, gels, and aerosols.
❓ How do you separate mixtures at home?
Common household separation methods include filtration (coffee brewing), evaporation (salt from salt water), decantation (oil from water), magnetic separation (iron from cereal), and crystallization (growing salt or sugar crystals). The method depends on the mixture type and component properties.
❓ What mixture is blood?
Blood is a complex suspension containing red blood cells, white blood cells, and platelets suspended in plasma (which itself is a solution of proteins, nutrients, and other dissolved substances). The cellular components can be separated through centrifugation, demonstrating suspension behavior.
❓ Is air a homogeneous mixture?
Yes, air is a homogeneous gaseous mixture containing approximately 78% nitrogen, 21% oxygen, and trace amounts of argon, carbon dioxide, and water vapor. Under normal conditions, these gases are uniformly distributed, making air a true solution in the gas phase.
❓ What type of mixture is milk?
Milk is an emulsion – specifically an oil-in-water emulsion where fat globules are dispersed in water with natural proteins acting as emulsifiers. It’s also technically a colloid due to the intermediate size of the fat particles and demonstrates the Tyndall effect.
❓ Can temperature change mixture types?
Temperature significantly affects mixture behavior and can cause type transitions. Heating can increase solubility (converting suspensions to solutions), break emulsions, or cause phase changes. Cooling may cause precipitation (solutions becoming suspensions) or solidify liquid mixtures.
❓ What’s the difference between a mixture and a compound?
Mixtures involve physical combinations where components retain individual properties and can be separated by physical methods. Compounds form through chemical bonding, creating new substances with entirely different properties that require chemical reactions for separation. Water (H₂O) is a compound, while salt water is a mixture.
❓ How do you identify unknown mixtures in the lab?
Use systematic identification: 1) Visual inspection for uniformity and phases, 2) Tyndall effect test for colloid identification, 3) Settling test for suspension detection, 4) Filtration test for particle size assessment, 5) Advanced analysis (spectroscopy, microscopy) for definitive identification.
❓ What role do mixtures play in environmental issues?
Mixtures are central to environmental challenges and solutions. Pollution often involves harmful mixture combinations requiring sophisticated separation techniques. Water treatment uses mixture science principles for purification. Air quality management addresses aerosol mixtures. Recent research shows mixture effects can be more harmful than individual components.
❓ Are there mixtures in space?
Yes, space contains various mixture types including stellar atmospheres (gaseous mixtures), cosmic dust (aerosol-like particle suspensions), interstellar medium (extremely dilute gas and particle mixtures), and planetary atmospheres (complex gas mixtures with unique compositions).
📚 Additional Resources & References
🔬 Scientific Databases
- Royal Society of Chemistry – Latest research publications
- American Chemical Society – Professional resources and journals
- PubMed Chemistry Database – Recent research articles
🏭 Industry Standards
- ASTM International – Mixture analysis standards
- FDA Guidelines – Pharmaceutical mixtures
- EPA Methods – Environmental analysis
Conclusion
Understanding the seven types of chemical mixtures – solutions, colloids, suspensions, emulsions, foams, gels, and aerosols – provides essential knowledge that spans chemistry, biology, engineering, and environmental science. From the morning coffee demonstrating solution principles to cutting-edge medical treatments using sophisticated colloidal systems, these mixture types form the foundation of countless natural processes and technological applications.
Recent research breakthroughs, including AI-driven environmental analysis and discoveries about cumulative mixture toxicity effects, continue advancing our understanding of these complex systems. The fundamental distinction between homogeneous and heterogeneous mixtures helps predict behavior and select appropriate analytical and separation methods, while understanding particle size ranges enables precise classification and treatment optimization.
Whether you’re a chemistry student mastering fundamental concepts, a professional working in pharmaceuticals or environmental science, or simply curious about the molecular world surrounding us, this comprehensive knowledge of mixture science provides invaluable insights into how substances interact and can be manipulated for beneficial purposes.
As we face future challenges in sustainability, personalized medicine, and advanced materials development, the principles governing chemical mixtures will remain central to developing innovative solutions. The integration of artificial intelligence, nanotechnology, and green chemistry approaches promises exciting developments in mixture science applications.
🎯 Key Action Steps:
- Practice identification using everyday examples around your home or laboratory
- Apply separation techniques to solve real-world problems
- Stay updated with latest research through scientific databases
- Join professional communities for continued learning and networking
- Share knowledge to advance mixture science education globally
The next time you observe milk in your cereal, appreciate smartphone material engineering, or benefit from precisely formulated medications, remember you’re experiencing practical applications of mixture science – a field continuously evolving to meet humanity’s needs while remaining grounded in these fundamental principles we’ve explored.