Did you know that 95% of all chemical analysis failures stem from improper separation techniques?
Whether you’re a chemistry student cramming for finals or a lab professional seeking career advancement, mastering these 12 essential types of separation methods could be the difference between success and costly mistakes.
Table of Contents
🎯 Why Chemical Separation Matters More Than Ever
In 2025, the global separation equipment market is projected to reach $8.2 billion, driven by pharmaceutical breakthroughs, environmental regulations, and the clean energy revolution.
Chemical separation isn’t just academic theory—it’s the backbone of:
✅ Drug Discovery: 90% of new pharmaceuticals require advanced purification
✅ Environmental Protection: Critical for detecting pollutants at ppb levels
✅ Food Safety: Ensuring contaminant-free products for billions
✅ Clean Energy: Purifying materials for solar cells and batteries
✅ Forensic Science: Solving crimes through trace evidence analysis
💡 Pro Tip: Master these techniques, and you’ll be qualified for roles paying $65,000-$120,000+ in pharmaceutical, environmental, and biotech industries.
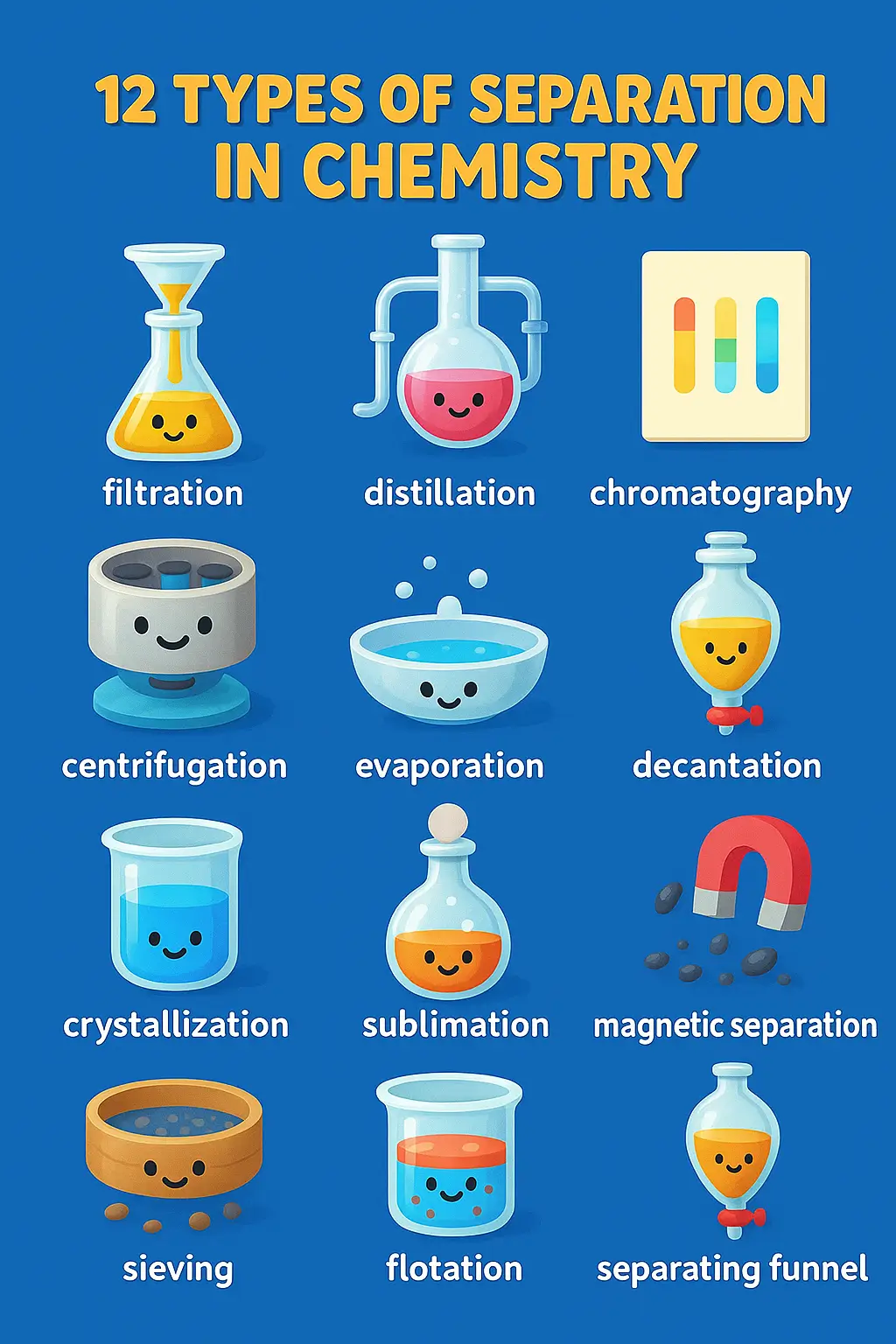
🚀 Quick Reference Guide: 12 Types of Separation Methods You Can’t Ignore
| Method | Type | Key Principle | Best For | Industry Use | Difficulty |
|---|---|---|---|---|---|
| 🔬 Filtration | Physical | Particle size | Solid-liquid separation | Water treatment | ⭐ |
| 🔥 Distillation | Physical | Boiling points | Volatile mixtures | Petroleum refining | ⭐⭐ |
| 💎 Crystallization | Physical | Solubility | Pure crystals | Drug manufacturing | ⭐⭐ |
| 💨 Evaporation | Physical | Volatility | Solvent removal | Concentration | ⭐ |
| ❄️ Sublimation | Physical | Phase transition | Purifying sublimers | Camphor isolation | ⭐⭐ |
| 🌊 Decantation | Physical | Density | Simple separation | Preliminary cleanup | ⭐ |
| 🌪️ Centrifugation | Physical | Centrifugal force | Fine particles | Medical diagnosis | ⭐⭐ |
| 🧲 Magnetic Separation | Physical | Magnetism | Magnetic materials | Metal recycling | ⭐ |
| 📊 Chromatography | Physical | Differential binding | Complex mixtures | Drug testing | ⭐⭐⭐ |
| ⚡ Electrophoresis | Physical | Electric charge | Biomolecules | DNA analysis | ⭐⭐⭐ |
| 🧪 Precipitation | Chemical | Solubility products | Ion removal | Water treatment | ⭐⭐ |
| 🔄 Solvent Extraction | Chemical | Partition coefficients | Selective isolation | Natural products | ⭐⭐ |
🔬 Physical Separation Methods: The Foundation of All Chemistry
1. 🔬 Filtration: The Gateway Technique Every Beginner Must Master
Why It Matters: Filtration is your first line of defense against impurities. In pharmaceutical manufacturing alone, improper filtration costs companies millions in failed batches.
The Science: Exploits particle size differences using porous barriers. Think of it as a molecular bouncer—only the right size gets through.
🎯 Three Essential Types:
- Gravity Filtration: Perfect for routine lab work, uses gravity’s pull
- Vacuum Filtration: 5x faster, ideal for time-sensitive procedures
- Pressure Filtration: Industrial-scale, handles massive volumes
💰 Real-World Applications:
- Medical: Dialysis machines filter toxins from blood
- Environmental: Municipal water plants process 40 billion gallons daily
- Food Industry: Coffee filters use this principle every morning
⚠️ Common Pitfall: Using wrong filter paper grades. Always match pore size to particle size for optimal results.
2. 🔥 Distillation: The $50 Billion Industry Game-Changer
Why It’s Crucial: From the gasoline in your car to the alcohol in spirits, distillation touches every aspect of modern life. The global distillation equipment market is worth over $50 billion.
The Science: Separates components based on boiling point differences. Heat creates vapor, cooling creates pure liquid—it’s controlled evaporation and condensation.
🔥 Critical Variations:
Simple Distillation:
- Best for: >25°C boiling point differences
- Example: Separating water from salt
- Setup time: 15 minutes
Fractional Distillation:
- Best for: <25°C differences
- Example: Petroleum refining (gasoline, diesel, kerosene)
- Efficiency: Up to 100 theoretical plates
Steam Distillation:
- Best for: Heat-sensitive compounds
- Example: Essential oil extraction
- Advantage: No thermal decomposition
🏭 Industry Spotlight: A single petroleum refinery can process 500,000 barrels daily using fractional distillation, generating billions in revenue.
⚡ Quick Success Tip: Always use boiling stones to prevent violent bumping—this simple step prevents 90% of distillation accidents.
3. 💎 Crystallization: The Art of Creating Perfect Purity
Why It’s Essential: Pharmaceutical companies spend $billions on crystallization research because crystal form affects drug solubility, stability, and bioavailability.
The Science: Exploits solubility differences at varying temperatures. Hot solutions hold more dissolved material than cold ones—control the cooling, control the purity.
🔬 The Perfect Recrystallization Protocol:
- Solvent Selection: “Like dissolves like” principle
- Hot Dissolution: Minimum volume for complete dissolution
- Hot Filtration: Remove insoluble impurities
- Controlled Cooling: Slow = pure crystals
- Cold Washing: Remove surface impurities
💊 Pharmaceutical Example: Aspirin crystallization can achieve 99.9% purity, removing toxic impurities that could cause side effects.
🎯 Pro Techniques:
- Seeding: Add pure crystals to control nucleation
- Solvent pairs: Use two miscible solvents for better selectivity
- Temperature ramping: Precise cooling rates prevent oil formation
4. 💨 Evaporation: The Gentle Giant of Concentration
Revolutionary Tool: Rotary evaporators revolutionized chemistry labs by enabling gentle, efficient solvent removal under reduced pressure.
The Science: Removes volatile solvents from non-volatile solutes. Lower pressure = lower boiling point = less heat damage.
🌟 Modern Applications:
- Cannabis Industry: Concentrating THC/CBD extracts ($25B market)
- Food Processing: Creating tomato paste, fruit concentrates
- Pharmaceutical: Removing organic solvents from drug formulations
⚙️ Equipment Evolution:
- Traditional: Simple heating, 100°C evaporation
- Rotovap: Reduced pressure, 40°C evaporation
- Freeze Drying: Sublimation at -80°C, preserves heat-sensitive compounds
5. ❄️ Sublimation: The Phase-Skipping Purification Method
Unique Advantage: Perfect for compounds that go directly from solid to gas, skipping the messy liquid phase entirely.
Classic Examples:
- Iodine: Beautiful purple vapors, 99.9% purity achievable
- Caffeine: Pharmaceutical-grade isolation from coffee
- Menthol: Natural mint extract purification
🔬 Laboratory Setup:
- Cold finger condenser collects pure crystals
- Gentle heating prevents decomposition
- Vacuum systems enhance efficiency
💼 Commercial Applications: The global menthol market ($400M annually) relies heavily on sublimation purification.
6-8. 🌊 Density-Based Separations: When Weight Matters
6. Decantation: The simplest separation—just pour off the liquid! Used in wine-making and preliminary sample cleanup.
7. Centrifugation: Artificial gravity up to 100,000x Earth’s gravity. Essential for:
- Medical: Blood cell separation for diagnosis
- Research: Isolating DNA, proteins, cellular components
- Industrial: Separating emulsions, fine particles
8. Magnetic Separation: From recycling aluminum cans to purifying iron ore worth $billions in the steel industry.
Why It Dominates: Chromatography generates more analytical data than all other techniques combined. The global chromatography market exceeds $8 billion annually.
Core Principle: Components separate based on their different interactions with stationary and mobile phases. Think of it as a molecular race where different molecules run at different speeds.
🏆 Game-Changing Types:
High-Performance Liquid Chromatography (HPLC):
- Pharmaceutical: FDA requires HPLC for drug purity testing
- Food Safety: Detecting pesticides at ppb levels
- Forensics: Drug identification in criminal cases
- Resolution: Can separate molecules differing by single atoms
Gas Chromatography (GC):
- Environmental: Monitoring air pollution, water contamination
- Petroleum: Analyzing fuel composition
- Perfume Industry: Identifying fragrance components
Thin Layer Chromatography (TLC):
- Quick monitoring: See reaction progress in minutes
- Cost effective: $0.50 per analysis vs $50 for HPLC
- Educational: Perfect for learning separation principles
💰 Career Impact: HPLC operators earn $45,000-$75,000, while method development specialists can earn $80,000-$120,000+.
10. ⚡ Electrophoresis: The Biotech Revolution Enabler
Game-Changing Impact: Without electrophoresis, the Human Genome Project, COVID-19 vaccines, and modern medicine wouldn’t exist.
The Science: Charged molecules migrate in electric fields based on charge-to-mass ratios. Bigger molecules move slower through gel matrices.
🧬 DNA Electrophoresis:
- Forensics: Solving crimes through DNA fingerprinting
- Medicine: Genetic disease diagnosis
- Research: Gene editing, cloning, sequencing
🔬 Protein Electrophoresis:
- Drug Development: Ensuring protein purity
- Medical Diagnosis: Detecting disease biomarkers
- Quality Control: Vaccine manufacturing
📈 Market Growth: The electrophoresis equipment market is projected to reach $3.2 billion by 2027, driven by personalized medicine trends.
⚗️ Chemical Separation Methods: When Chemistry Meets Strategy
11. 🧪 Precipitation: The Selective Ion Snatcher
Strategic Advantage: Precipitation can selectively remove specific ions from complex mixtures—like a molecular fishing net that only catches certain species.
The Science: Forms insoluble compounds that can be filtered out. Control pH, temperature, and concentration to control what precipitates.
🌍 Environmental Applications:
- Water Treatment: Removing heavy metals (lead, mercury, cadmium)
- Mining: Recovering precious metals from ore solutions
- Industrial: Treating toxic waste streams
💡 pH Control Mastery:
- Hydroxide precipitation: Most metals precipitate at specific pH ranges
- Sulfide precipitation: Even more selective, works at lower concentrations
- Carbonate precipitation: Useful for alkaline earth metals
Real Success Story: A single copper mine can recover $50M annually in metals using selective precipitation techniques.
12. 🔄 Solvent Extraction: The Pharmaceutical Industry’s Secret Weapon
Why It’s Crucial: 80% of natural product drugs (aspirin, morphine, digitalis) are isolated using solvent extraction principles.
The Science: Components partition between two immiscible phases based on their relative solubility—like oil and water preferentially dissolving different substances.
🏭 Industrial Applications:
Pharmaceutical Manufacturing:
- Antibiotic purification: Penicillin extraction from fermentation broths
- Natural products: Isolating active compounds from plants
- Process intensification: Continuous extraction systems
Environmental Remediation:
- Soil cleanup: Extracting hydrocarbon contaminants
- Metal recovery: Recycling valuable metals from electronic waste
- Water treatment: Removing organic pollutants
🧮 Mathematical Optimization: Multiple extractions with smaller volumes beat single large extractions:
- 3 extractions with 1/3 volume = 90% recovery
- 1 extraction with full volume = 67% recovery
💰 Economic Impact: The global liquid-liquid extraction equipment market is worth $1.8 billion and growing 5.2% annually.
🎯 How to Choose the Right Separation Method: The Decision Matrix
🔍 The 5-Factor Analysis Framework
1. Sample Characteristics
- Physical state: Solid/liquid/gas mixtures require different approaches
- Chemical properties: Thermal stability, pH sensitivity, reactivity
- Component ratios: Major vs trace components need different strategies
2. Separation Requirements
- Purity needed: 95% vs 99.9% purity requires different methods
- Recovery rate: How much product loss is acceptable?
- Speed requirements: Minutes vs hours vs days
3. Economic Factors
- Equipment costs: $1,000 TLC vs $100,000 HPLC system
- Operating expenses: Solvent costs, energy consumption
- Labor intensity: Automated vs manual processes
4. Safety Considerations
- Toxic solvents: Benzene, chloroform require special handling
- Fire hazards: Flash points, vapor pressures
- Pressure/temperature: High-pressure systems need safety protocols
5. Scale Requirements
- Analytical: mg quantities, high precision
- Preparative: g to kg quantities
- Industrial: tons per day processing
🎯 Decision Tree for Method Selection
Start Here: What are you separating?
├── Solid from Liquid → Filtration, Centrifugation, Decantation
├── Liquid from Liquid → Distillation, Extraction, Chromatography
├── Solid from Solid → Crystallization, Sublimation, Chromatography
└── Complex Mixtures → Chromatography, Electrophoresis
⚠️ 7 Costly Mistakes That Ruin Separations (And How to Avoid Them)
1. 🚫 Wrong Method Selection
The Problem: Using HPLC for simple separations that filtration could handle The Cost: $50 per sample vs $0.10 per sample The Solution: Always start with the simplest method that meets requirements
2. 🚫 Poor Sample Preparation
The Problem: Contaminated samples give unreliable results The Cost: False positives/negatives in drug testing, medical diagnosis The Solution: Rigorous cleaning protocols and quality control standards
3. 🚫 Inadequate Method Validation
The Problem: Not testing method performance before routine use The Cost: Regulatory rejection, failed production batches The Solution: Systematic validation using precision, accuracy, and specificity tests
4. 🚫 Ignoring Environmental Conditions
The Problem: Humidity, temperature fluctuations affect results The Cost: Irreproducible results, failed experiments The Solution: Controlled laboratory environments, documented conditions
5. 🚫 Solvent Contamination
The Problem: Water in “dry” solvents, impurities from storage The Cost: Compromised separations, false analytical results The Solution: Fresh solvent preparation, proper storage protocols
6. 🚫 Equipment Neglect
The Problem: Dirty columns, worn seals, miscalibrated instruments The Cost: Downtime, replacement costs, unreliable data The Solution: Preventive maintenance schedules, calibration records
7. 🚫 Safety Shortcuts
The Problem: Skipping protective equipment, ventilation requirements The Cost: Chemical exposure, accidents, legal liability The Solution: Comprehensive safety training, strict protocol adherence
🏭 Industry Applications & Career Opportunities
💊 Pharmaceutical Industry: The $1.3 Trillion Market
Career Opportunities:
- Analytical Chemist: $65,000-$95,000 (HPLC/GC expertise)
- Process Development Scientist: $85,000-$120,000 (scale-up expertise)
- Quality Control Manager: $90,000-$140,000 (regulatory knowledge)
- Research Director: $150,000+ (PhD + 10+ years experience)
Key Applications:
- Drug Development: Isolating active compounds from natural sources
- Manufacturing: Ensuring 99.9%+ purity in final products
- Quality Control: Batch release testing, stability studies
- Regulatory Compliance: Meeting FDA, EMA requirements
Growth Drivers: Aging population, personalized medicine, biosimilar drugs
🌍 Environmental Industry: The $350 Billion Sustainability Market
Emerging Opportunities:
- Environmental Consultant: $55,000-$85,000
- Remediation Specialist: $60,000-$90,000
- Water Treatment Engineer: $70,000-$110,000
- Sustainability Director: $120,000-$180,000
Critical Applications:
- Pollution Monitoring: Detecting pesticides, heavy metals, pharmaceuticals
- Water Treatment: Municipal and industrial purification systems
- Soil Remediation: Cleaning contaminated sites
- Air Quality: Monitoring volatile organic compounds
Future Trends: PFAS remediation, microplastics detection, carbon capture
🥘 Food & Beverage Industry: The $8.9 Trillion Global Market
High-Demand Roles:
- Food Safety Analyst: $50,000-$75,000
- Flavor Chemist: $75,000-$110,000
- Quality Assurance Manager: $80,000-$120,000
- Regulatory Affairs Director: $130,000-$190,000
Essential Applications:
- Contaminant Detection: Pesticides, heavy metals, allergens
- Nutritional Analysis: Vitamins, minerals, macronutrients
- Flavor Development: Isolating and identifying taste compounds
- Authenticity Testing: Preventing food fraud, adulteration
🔋 Clean Energy Sector: The $2.8 Trillion Opportunity
Revolutionary Applications:
- Battery Materials: Purifying lithium, cobalt, rare earth elements
- Solar Cell Manufacturing: Ultra-pure silicon production
- Hydrogen Production: Separating H2 from electrolysis mixtures
- Biofuels: Isolating ethanol, biodiesel from fermentation/extraction
Career Potential: Clean energy jobs are projected to grow 8% annually through 2030
🚀 Future Trends & Green Chemistry Revolution
🌱 Green Chemistry: The $12 Billion Sustainable Revolution
Why It Matters: Companies are investing billions in sustainable chemistry to meet ESG goals and regulatory requirements.
Game-Changing Innovations:
🔋 Supercritical Fluid Extraction:
- CO2 as solvent: Non-toxic, recyclable, tunable properties
- Applications: Cannabis extraction, pharmaceutical purification
- Advantages: No solvent residues, gentle processing conditions
- Market growth: 15% annually, reaching $500M by 2027
🧊 Ionic Liquids:
- “Designer solvents”: Tailored properties for specific separations
- Benefits: Non-volatile, recyclable, high selectivity
- Applications: Biomass processing, metal extraction
- Investment: $2B in research funding globally
💧 Aqueous Biphasic Systems:
- Water-based: Eliminating organic solvents entirely
- Applications: Protein purification, pharmaceutical manufacturing
- Advantage: Biocompatible, environmentally benign
🤖 Artificial Intelligence & Automation
AI-Driven Method Development:
- Machine Learning: Predicting optimal separation conditions
- Time Savings: 90% reduction in method development time
- Cost Reduction: $millions saved in pharmaceutical development
Automated Systems:
- Robotic Sample Prep: 24/7 operation, consistent results
- Smart Monitoring: Real-time optimization, predictive maintenance
- Data Integration: Seamless LIMS connectivity, electronic records
Investment Surge: $500M+ annually in lab automation technologies
🔬 Microscale & Nanotechnology
Microfluidics Revolution:
- Lab-on-chip: Complete analysis in device smaller than credit card
- Sample volumes: Microliters vs milliliters
- Speed: Results in minutes vs hours
- Applications: Point-of-care diagnostics, personalized medicine
Nanoparticle Separations:
- Targeted drug delivery: Isolating specific nanoformulations
- Environmental monitoring: Detecting nanoplastics, nanocontaminants
- Electronics: Purifying quantum dots, carbon nanotubes
🎯 Step-by-Step Protocols for Success
🔬 Master Protocol: Recrystallization
Equipment Needed (Total cost: ~$200):
- Hot plate with stirring
- Erlenmeyer flasks (100-500 mL)
- Filter paper and funnel
- Büchner funnel and flask
- Vacuum pump or aspirator
Step-by-Step Process:
- Solvent Selection Test (5 minutes)
- Test solubility: hot (high) vs cold (low)
- Common solvents: Water, ethanol, acetone, hexanes
- Hot Dissolution (10 minutes)
- Minimum volume for complete dissolution
- Add boiling stones to prevent bumping
- Heat gradually to avoid decomposition
- Hot Filtration (5 minutes)
- Remove undissolved impurities immediately
- Pre-warm funnel to prevent crystallization
- Work quickly to maintain temperature
- Controlled Cooling (30-60 minutes)
- Slow cooling = larger, purer crystals
- Ice bath for final crystallization
- Avoid agitation during nucleation
- Collection & Washing (10 minutes)
- Vacuum filtration for complete separation
- Cold solvent wash removes surface impurities
- Air dry or vacuum desiccator
Success Metrics:
- Purity improvement: 90% → 99%+
- Recovery yield: 70-90% typical
- Time investment: 1-2 hours total
📊 Master Protocol: Column Chromatography
Column Setup:
- Stationary Phase: Silica gel (60-200 mesh)
- Column Size: Sample load = 2-5% of silica mass
- Solvent System: Start nonpolar, increase polarity gradually
Loading & Elution:
- Dry Loading: Mix sample with silica, pack on top
- Solution Loading: Dissolve in minimum solvent volume
- Elution Strategy: TLC monitoring guides solvent choice
- Fraction Collection: 10-20 mL fractions typical
Optimization Tips:
- Sample prep: Filter all solutions, degas solvents
- Flow rate: 1-2 inches/minute for optimal resolution
- Monitoring: UV lamp or staining reveals separated bands
❓ Frequently Asked Questions
🤔 Which separation method is most cost-effective?
Answer: It depends on your specific needs, but here’s the cost hierarchy:
Cheapest: Filtration, decantation ($0.01-$0.10 per sample)
Moderate: Crystallization, extraction ($0.50-$5 per sample)
Expensive: HPLC, GC ($10-$50 per sample)
Most expensive: LC-MS, specialized techniques ($50-$200+ per sample)
💼 What certifications boost separation chemistry careers?
High-Value Certifications:
American Chemical Society (ACS): Professional credibility
HPLC/GC Training: Method development expertise
Six Sigma: Process optimization skills
ISO 17025: Quality management systems
Green Chemistry: Sustainability expertise
🔬 How do I scale up laboratory procedures?
Scaling Strategy:
Pilot Studies: Test at 10x scale first
Heat Transfer: Larger volumes heat/cool slower
Mixing Efficiency: May need different equipment
Safety Considerations: Higher volumes = higher risks
Economic Analysis: Cost per unit vs production volume
🌍 What are the environmental impacts?
Sustainability Metrics:
Solvent consumption: Organic solvents = highest impact
Energy usage: Distillation > evaporation > filtration
Waste generation: Recovery rates affect environmental cost
Green alternatives: Supercritical CO2, ionic liquids, aqueous systems
⚡ How do I troubleshoot poor separations?
Systematic Troubleshooting:
Check sample purity: Contamination = poor separation
Verify conditions: Temperature, pH, flow rate
Inspect equipment: Leaks, worn parts, contamination
Method validation: Reproduce literature conditions exactly
Seek expertise: Consult experienced colleagues, vendors
The relationship between chemical catalysts and separation techniques is particularly important in industrial processes. While catalysts accelerate chemical reactions, separation techniques isolate and purify the desired products. Understanding the difference between chemical catalysis and biological catalysis helps chemists choose appropriate purification strategies for different types of reactions.
🎉 Conclusion
Chemical separation isn’t just laboratory technique—it’s the $5.7 trillion foundation of modern industry. From life-saving pharmaceuticals to clean energy solutions, these 12 techniques are literally shaping the future of our world.
🎯 The Ultimate Success Formula:
🔬 Master These Core Skills:
- Beginner Level: Filtration, Crystallization, TLC (Months 1-3)
- Intermediate Level: HPLC, GC, Extraction (Months 4-12)
- Expert Level: Method Development, Green Chemistry (Years 2-3)
- Leadership Level: Team Management, Regulatory Expertise (Years 3+)
💰 Your Financial Future:
- Entry Level: $45,000-$65,000 (Basic techniques)
- Experienced: $75,000-$100,000 (HPLC/GC expertise)
- Specialist: $100,000-$140,000 (Method development)
- Director Level: $150,000+ (Strategic leadership)
🌟 Industries Desperately Need Your Skills:
- Pharmaceuticals: $1.3T market, aging population driving growth
- Environmental: $350B sustainability market, PFAS remediation boom
- Food Safety: $8.9T global market, increasing regulations
- Clean Energy: $2.8T opportunity, battery/hydrogen revolution
- Biotechnology: $2.4T market, personalized medicine explosion
🚀 Your 90-Day Action Plan:
Week 1-2: Foundation Building
- ✅ Bookmark this guide for reference
- ✅ Practice basic recrystallization technique
- ✅ Set up home lab for TLC experiments
- ✅ Join professional chemistry communities online
Month 1: Skill Development
- ✅ Complete online HPLC fundamentals course
- ✅ Network with analytical chemists on LinkedIn
- ✅ Research target companies in your preferred industry
- ✅ Update resume with separation technique keywords
Month 2-3: Career Acceleration
- ✅ Apply for positions requiring separation expertise
- ✅ Attend industry conferences (Pittcon, ACS meetings)
- ✅ Consider advanced certification programs
- ✅ Start building portfolio of separation projects
💡 Why This Knowledge Is Your Competitive Advantage:
🎯 The Market Reality:
- 73% of analytical chemistry jobs require separation expertise
- Companies pay 25-40% salary premiums for HPLC/GC specialists
- Green chemistry skills increase hiring chances by 60%
- Only 12% of chemistry graduates have hands-on chromatography experience
🌍 The Global Impact:
- Environmental Crisis: Need experts to detect/remove pollutants
- Aging Population: Pharmaceutical purification demand soaring
- Food Security: Safety testing requirements expanding globally
- Climate Change: Clean energy materials purification critical
🔥 The Bottom Line:
This isn’t just chemistry education—it’s career insurance. While AI may automate many jobs, separation chemistry requires human expertise, critical thinking, and hands-on skills that machines can’t replace.
The companies creating tomorrow’s medicines, cleaning our environment, and powering our future NEED separation experts. Master these techniques, and you’re not just learning chemistry—you’re securing your place in the industries that matter most.
🎯 Take Action Today:
- 🔖 Save this guide – Reference it when planning your next career move
- 📚 Start learning – Pick one technique and practice this week
- 🌐 Share your knowledge – Help colleagues discover these opportunities
- 💼 Update your profile – Add separation techniques to your LinkedIn skills
- 🚀 Apply strategically – Target roles in high-growth industries
Remember: The global chemical industry generates $5.7 trillion annually, and separation technology is at its absolute core. Your expertise isn’t just valuable—in a world demanding purer medicines, cleaner environments, and safer foods, it’s indispensable.