⚡ Quick Answer: The 7 Types of Chemical Bonds
Did you know? Every single substance around you—from the screen you’re reading this on to the air you’re breathing—exists because atoms have mastered the art of bonding.
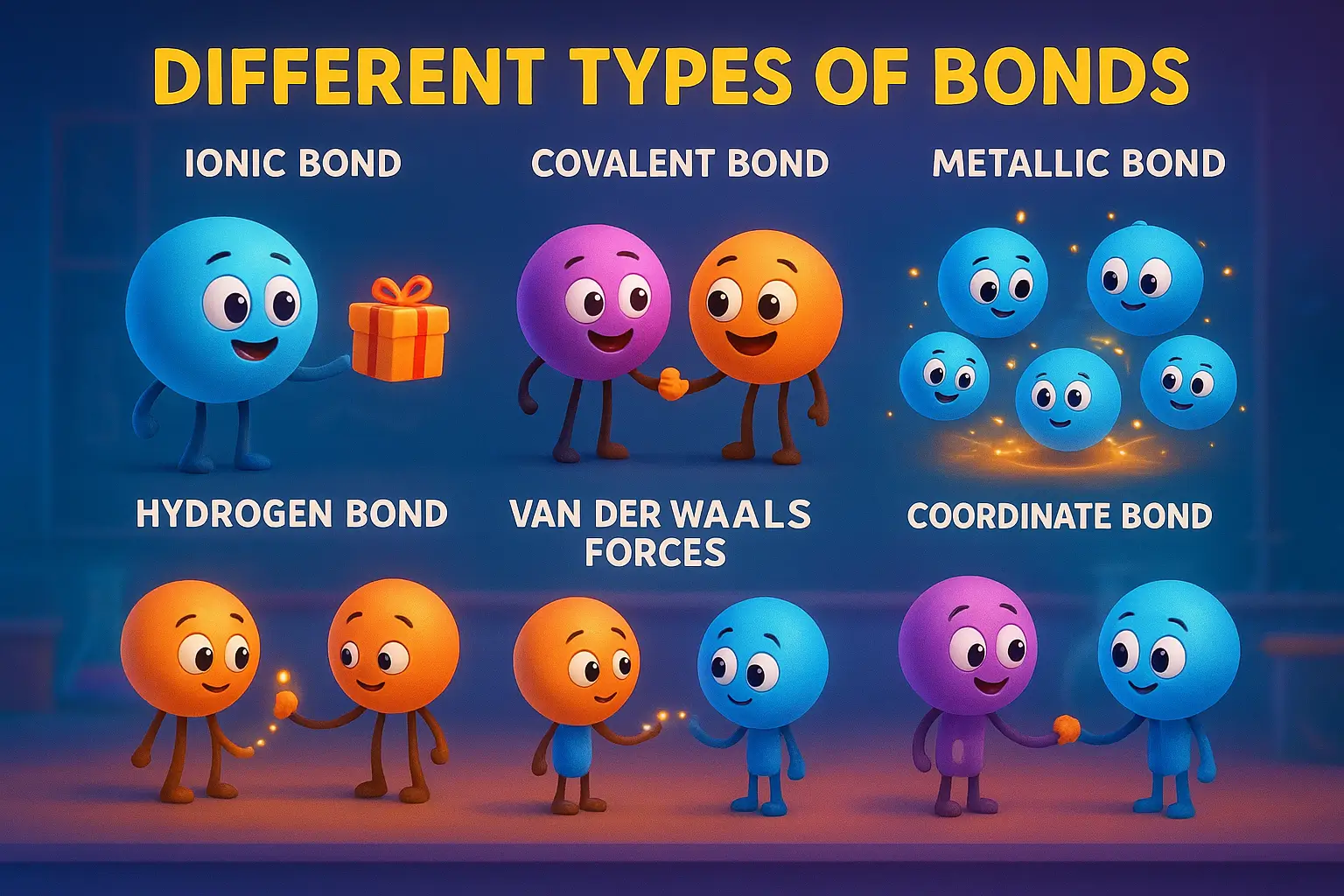
Need the answer fast? Here are the 7 types of chemical bonds that hold our world together:
- Ionic Bonds – Complete electron transfer between metals and non-metals (NaCl)
- Covalent Bonds – Electron sharing between non-metals (H₂O, CO₂)
- Metallic Bonds – Electron sea model in metals (copper, iron)
- Hydrogen Bonds – Special attraction involving H-F, H-O, H-N (DNA, proteins)
- Van der Waals Forces – Weak universal attractions (gecko feet adhesion)
- Dipole-Dipole Interactions – Attractions between polar molecules (acetone-water)
- Coordinate Bonds – One-way electron sharing (NH₄⁺, metal complexes)
🎯 Key Takeaway: These bonds range from strongest (ionic/covalent) to weakest (Van der Waals), each with unique properties that determine how materials behave in our world.
Want the complete explanation with examples, properties, and exam tips? Keep reading!
Table of Contents
What Are Chemical Bonds? Understanding the Basics
Quick Definition: Chemical bonds are attractive forces that hold atoms together in compounds and molecules.
The WHY Behind Chemical Bonding
Atoms aren’t social by nature—they bond for one reason: stability. Every atom wants to achieve the most stable electron configuration possible, typically by having complete outer electron shells (following the octet rule).
Key Players in Bonding:
- Valence electrons: The electrons in the outermost shell
- Electronegativity: An atom’s ability to attract electrons
- Energy considerations: Bonds form when the result is more stable than separate atoms
Pro Tip: Think of atoms like puzzle pieces—they only fit together in ways that make them more stable than they were alone.
Understanding what atoms are made of and the difference between atoms and molecules is crucial for mastering chemical bonding concepts.
1. Ionic Bonds – The Complete Electron Transfer
🎯 What You Need to Know:
- Definition: Complete transfer of electrons from metal to non-metal
- Key Players: Metals (lose electrons) + Non-metals (gain electrons)
- Result: Charged ions held together by electrostatic forces
How Ionic Bonds Form (Step-by-Step)
- Metal atoms lose valence electrons → become positive cations (Na⁺)
- Non-metal atoms gain electrons → become negative anions (Cl⁻)
- Opposite charges attract → ionic bond forms
- Crystal lattice structure develops for maximum stability
Classic Example: Sodium Chloride (NaCl) – Table Salt
- Na loses 1 electron → Na⁺
- Cl gains 1 electron → Cl⁻
- Electrostatic attraction = ionic bond
Properties That’ll Help You in Exams:
Properties of Ionic Compounds
Understanding the unique characteristics of ionic bonding
| Property | Explanation | Example |
|---|---|---|
| High melting/boiling points | Strong electrostatic forces need lots of energy to break | NaCl melts at 801°C |
| Electrical conductivity | Conducts when molten/dissolved (ions mobile) | Salt water conducts electricity |
| Solubility in water | Polar water molecules surround ions | Salt dissolves in water |
| Brittleness | Shifting structure brings like charges together | Crystals shatter when stressed |
Common Ionic Compounds You Should Know:
- NaCl (Sodium chloride) – Table salt
- CaF₂ (Calcium fluoride) – Optical materials
- MgO (Magnesium oxide) – Antacids
- KBr (Potassium bromide) – Photography
2. Covalent Bonds – The Sharing Masters
🎯 What You Need to Know:
- Definition: Atoms share electron pairs to achieve stability
- Key Players: Usually non-metals with non-metals
- Types: Single, double, triple bonds + polar/nonpolar variations
Types of Covalent Bonds (Strength Ranking)
Single Bonds (Weakest)
- 1 shared electron pair (C-C)
- Longest bond length
- Allow free rotation
- Most flexible
Double Bonds (Stronger)
- 2 shared electron pairs (C=C)
- Shorter than single bonds
- Prevent free rotation
- Create geometric isomers
Triple Bonds (Strongest)
- 3 shared electron pairs (C≡C)
- Shortest bond length
- Extremely strong
- Example: N≡N (explains nitrogen’s stability)
Polar vs Nonpolar Covalent Bonds
Nonpolar Covalent:
- Equal sharing of electrons
- Similar electronegativity values
- Example: H-H in hydrogen gas
- No charge separation
Polar Covalent:
- Unequal sharing of electrons
- Different electronegativity values
- Example: H-O in water (oxygen more electronegative)
- Creates partial charges (δ+ and δ-)
Properties of Covalent Compounds:
Properties of Molecular Compounds
Understanding the unique characteristics of molecular bonding
| Property | Why It Happens | Examples |
|---|---|---|
| Lower melting points | Weaker intermolecular forces | Water melts at 0°C vs NaCl at 801°C |
| Poor conductivity | Electrons aren’t mobile | Pure water doesn’t conduct |
| Variable solubility | “Like dissolves like” rule | Oil (nonpolar) doesn’t mix with water (polar) |
| Molecular structures | Form discrete molecules | H₂O, CH₄, CO₂ |
3. Metallic Bonds – The Electron Sea Model
🎯 What You Need to Know:
- Definition: Metal atoms share electrons in a delocalized “sea”
- Key Feature: Electrons move freely throughout the structure
- Result: Unique properties that define metals
The Electron Sea Model Explained
Think of metallic bonding like a swimming pool filled with electrons:
- Metal ions = Fixed structures around the pool
- Delocalized electrons = Water that flows freely
- Bonding = The “water” holds everything together
This model perfectly explains why metals have their unique properties.
Properties Explained by Metallic Bonding:
🔌 Electrical Conductivity
- Mobile electrons carry current efficiently
- Why it matters: Essential for all electronics
🔥 Thermal Conductivity
- Electrons transfer kinetic energy quickly
- Real-world use: Copper cookware, heat sinks
🔨 Malleability & Ductility
- Non-directional bonding allows atoms to slide
- Practical result: Can hammer into sheets, draw into wires
✨ Metallic Lustre
- Electron sea absorbs/re-emits light
- Creates: Characteristic shiny appearance
Everyday Examples:
- Copper wiring – Excellent conductor
- Aluminum foil – Malleable due to metallic bonding
- Steel structures – Strong yet workable
- Gold jewelry – Doesn’t corrode, maintains shine
4. Hydrogen Bonds – Nature’s Life Force
🎯 What You Need to Know:
- Definition: Special attraction between hydrogen and highly electronegative atoms
- Strength: Stronger than other intermolecular forces, weaker than covalent
- Biological importance: Essential for life as we know it
Formation Requirements (The F-O-N Rule)
Hydrogen bonds need three key components:
- Hydrogen atom bonded to F, O, or N (remember: F-O-N)
- Electronegative atom with lone pairs (the acceptor)
- Proper geometry for interaction
Why Hydrogen Bonds Are Life-Changing:
🧬 DNA Double Helix
- A-T pairs: 2 hydrogen bonds
- G-C pairs: 3 hydrogen bonds
- Function: Hold strands together, allow separation for replication
🧪 Protein Structure
- Secondary structure: α-helices and β-sheets
- Tertiary structure: Overall 3D shape
- Result: Determines protein function
💧 Water’s Unique Properties
- High boiling point: Should boil at -80°C based on size alone
- Surface tension: Allows insects to walk on water
- Ice floats: Less dense than liquid water
Types of Hydrogen Bonds:
| Type | Location | Example | Importance |
|---|---|---|---|
| Intermolecular | Between different molecules | Water-water | Liquid water properties |
| Intramolecular | Within same molecule | Protein folding | Maintains molecular shape |
5. Van der Waals Forces – The Universal Attraction
🎯 What You Need to Know:
- Also called: London dispersion forces
- Strength: Weakest intermolecular force
- Universality: Present between ALL atoms and molecules
- Importance: Despite weakness, they’re everywhere
How They Work (The Electron Dance)
- Electrons constantly move around atoms/molecules
- Temporary dipoles form due to electron movement
- Induced dipoles created in neighboring atoms
- Weak attraction results from these temporary charges
Factors Affecting Strength:
Molecular Size 📏
- Larger molecules = More electrons = Stronger forces
- Example: Boiling points increase from He to Rn
Molecular Shape 🔄
- Linear molecules generally stronger than spherical
- More surface contact = Stronger attraction
Polarizability ⚡
- Easily distorted electrons = Stronger temporary dipoles
- Heavier atoms typically more polarizable
Amazing Real-World Examples:
🦎 Gecko Feet
- Millions of tiny hairs maximize surface contact
- Van der Waals forces provide climbing ability
- No sticky substances needed!
⛽ Petroleum Products
- Different boiling points of hydrocarbons
- Separation in refineries based on dispersion force differences
6. Dipole-Dipole Interactions – Polar Attractions
🎯 What You Need to Know:
- Definition: Attractions between polar molecules
- Requirement: Molecules must have permanent dipoles
- Strength: Stronger than Van der Waals, weaker than hydrogen bonds
How Polar Molecules Attract
Polar molecules have permanent charge separations (dipoles):
- Partially positive end (δ+)
- Partially negative end (δ-)
- Opposite ends attract between molecules
Factors Affecting Strength:
- Dipole magnitude – How polar the molecule is
- Distance – Closer molecules attract more strongly
- Orientation – Proper alignment maximizes attraction
Important Applications:
🧪 Solvent Selection
- Polar solvents dissolve polar solutes
- Example: Acetone (polar) mixes with water (polar)
- Rule: “Like dissolves like”
💊 Drug Design
- Optimize interactions with biological targets
- Control solubility and absorption
- Enhance selectivity for specific receptors
Common Examples:
- HCl molecules – Polar due to electronegativity difference
- Acetone – Polar C=O bond creates dipole
- Ammonia (NH₃) – Polar N-H bonds
7. Coordinate Covalent Bonds – One-Way Electron Sharing
🎯 What You Need to Know:
- Also called: Dative bonds
- Special feature: Both electrons come from same atom
- Strength: Same as regular covalent bonds once formed
- Formation: Donor provides electrons, acceptor provides empty orbital
Formation Requirements:
Donor Atom Must Have:
- ✅ Lone pair of electrons to donate
- ✅ Ability to overlap orbitals with acceptor
Acceptor Atom Must Have:
- ✅ Empty orbital of appropriate energy
- ✅ Capacity for additional electrons
Essential Examples for Exams:
Ammonium Ion (NH₄⁺)
- Formation: NH₃ + H⁺ → NH₄⁺
- Process: Nitrogen lone pair bonds to H⁺
- Result: Fourth N-H bond via coordinate bonding
Metal Complexes
- Transition metals accept electron pairs from ligands
- Examples: [Cu(NH₃)₄]²⁺, [Fe(CN)₆]³⁻
- Applications: Biological systems, catalysis
Carbon Monoxide Complexes
- CO donates lone pair to metals
- Importance: Organometallic chemistry
- Warning: CO’s strong binding makes it toxic
Quick Reference Comparison Table
Types of Chemical Bonds
Understanding the different types of chemical bonding
| Bond Type | Strength | Formation Method | Key Properties | Common Examples |
|---|---|---|---|---|
| Ionic | Strong | Electron transfer | High MP/BP, conducts when molten | NaCl, MgO, CaF₂ |
| Covalent | Strong | Electron sharing | Directional, varied properties | H₂O, CH₄, CO₂ |
| Metallic | Variable | Electron sea | Conductive, malleable, shiny | Cu, Fe, Al |
| Hydrogen | Moderate | H bonded to F,O,N | Crucial for biology, high BP | DNA, proteins, water |
| Van der Waals | Weak | Temporary dipoles | Universal, size-dependent | Noble gases, hydrocarbons |
| Dipole-Dipole | Weak-Moderate | Permanent dipoles | Orientation dependent | HCl, acetone |
| Coordinate | Strong | One-way sharing | Forms complex ions | NH₄⁺, metal complexes |
How to Identify Bond Types: Your Step-by-Step Guide
🔍 Step 1: Examine the Elements
| Element Combination | Most Likely Bond Type |
|---|---|
| Metal + Non-metal | Ionic |
| Non-metal + Non-metal | Covalent |
| Metal + Metal | Metallic |
🔍 Step 2: Check Electronegativity Differences
| Electronegativity Difference | Bond Type |
|---|---|
| > 1.7 | Ionic |
| 0.4 – 1.7 | Polar Covalent |
| < 0.4 | Nonpolar Covalent |
🔍 Step 3: Look for Special Cases
- H bonded to F, O, or N → Hydrogen bonding possible
- Lone pairs near empty orbitals → Coordinate bonding possible
- Polar molecules → Dipole-dipole interactions
- All molecules → Van der Waals forces present
Practice Examples:
Water (H₂O)
- Elements: Non-metal + Non-metal ✓
- Electronegativity: O (3.4) vs H (2.2) = 1.2 difference
- Result: Polar covalent bonds + hydrogen bonding
Sodium Chloride (NaCl)
- Elements: Metal + Non-metal ✓
- Electronegativity: Cl (3.0) vs Na (0.9) = 2.1 difference
- Result: Ionic bonding
Methane (CH₄)
- Elements: Non-metal + Non-metal ✓
- Electronegativity: C (2.5) vs H (2.2) = 0.3 difference
- Result: Nonpolar covalent bonds
Real-World Applications That’ll Blow Your Mind
🏭 Materials Science Revolution
Smart Polymers
- Covalent bonding patterns determine flexibility
- Applications: Self-healing materials, shape-memory polymers
- Future: Programmable materials that respond to stimuli
Semiconductor Technology
- Precise covalent bonding in silicon crystals
- Doping control creates electronic properties
- Result: Every computer chip in existence
Superconductor Development
- Understanding electron behavior in metallic systems
- Goal: Room-temperature superconductors
- Impact: Revolutionary energy transmission
🧬 Biological Systems Mastery
Enzyme Design
- Multiple bond types create specific active sites
- Precision: One wrong bond = non-functional enzyme
- Applications: Industrial catalysis, medical treatments
Drug Discovery
- Optimize all bond types for target interaction
- Balance: Strong enough binding, easy metabolism
- Success stories: COVID-19 vaccines, cancer treatments
Cell Membrane Engineering
- Covalent bonds within lipid molecules
- Intermolecular forces between molecules
- Result: Selective permeability for life
⚗️ Industrial Chemistry Breakthroughs
Catalyst Design
- Coordinate bonding between metals and reactants
- Efficiency: Speed up reactions, reduce energy costs
- Examples: Petroleum refining, pharmaceutical synthesis
Advanced Adhesives
- Multiple intermolecular forces for specific materials
- Innovation: Reversible adhesives, underwater glues
- Applications: Medical devices, aerospace
Corrosion Prevention
- Understanding metallic bonding vulnerabilities
- Solutions: Protective alloys, smart coatings
- Savings: Billions in infrastructure protection
Frequently Asked Questions
Q: Which type of chemical bond is the strongest?
A: Among intramolecular bonds, covalent and ionic bonds are generally the strongest, with covalent triple bonds being particularly strong. However, the “strongest” depends on context – metallic bonds can be incredibly strong in bulk materials.
Q: Why is hydrogen bonding so important for life?
A: Hydrogen bonds are perfectly balanced – strong enough to provide stability (DNA structure, protein folding) but weak enough to be broken and reformed easily (DNA replication, enzyme function). This flexibility is essential for biological processes.
Q: How do I remember the difference between polar and nonpolar covalent bonds?
A: Think of electronegativity difference: Similar electronegativity = Fair sharing (nonpolar), Different electronegativity = Unfair sharing (polar). A difference > 0.4 usually indicates polar character.
Q: What makes metallic bonding unique?
A: The electron sea model! Unlike other bonds where electrons are localized between specific atoms, metallic bonding involves delocalized electrons that move freely throughout the entire structure. This explains all the unique metallic properties.
Q: Can a molecule have multiple types of bonds?
A: Absolutely! Complex molecules often contain multiple bond types. For example, proteins have covalent bonds in their backbone, hydrogen bonds for secondary structure, and Van der Waals forces for overall stability.
Q: How do I identify coordinate bonds in molecules?
A: Look for situations where one atom has lone pairs (like NH₃, H₂O) interacting with atoms that have empty orbitals (like H⁺, metal ions). The resulting bond uses electrons from only one of the original atoms.
Q: Why don’t noble gases form bonds easily?
A: Noble gases already have complete outer electron shells (stable octet), so they have little driving force to form bonds. However, under extreme conditions, some can form compounds (like XeF₄).
Q: What’s the relationship between bond length and bond strength?
A: Generally, shorter bonds are stronger. Triple bonds < Double bonds < Single bonds in length, but Triple bonds > Double bonds > Single bonds in strength. This is due to more electron pairs being shared in shorter spaces.
Master Chemical Bonding: Your Next Steps
Congratulations! You’ve just mastered the 7 essential types of chemical bonds that form the foundation of all chemistry. Here’s what you should do next:
✅ Immediate Actions:
- Bookmark this guide for quick reference during studies
- Practice identifying bonds in everyday materials around you
- Test your knowledge with the examples provided
- Share with classmates who are struggling with bonding concepts
📚 Deepen Your Understanding:
- Explore what atoms are made of for foundational knowledge
- Learn about the difference between atoms and molecules
- Understand how these concepts apply to real-world chemistry
🎯 Exam Success Strategy:
- Create flashcards with bond types and properties
- Practice past paper questions on chemical bonding
- Draw diagrams showing electron arrangements for each bond type
- Memorize the comparison table for quick recall
🌟 Beyond the Basics:
Understanding these 7 bond types is just the beginning. Every advanced chemistry concept – from reaction mechanisms to materials science – builds upon these fundamental principles. Master them now, and you’ll find the rest of chemistry becomes much more logical and predictable.
Remember: Chemistry isn’t about memorizing facts; it’s about understanding the patterns that govern how matter behaves. These bonding concepts are your key to unlocking those patterns.
Ready to dive deeper into chemistry? Explore our comprehensive collection of chemistry guides and resources designed to make complex topics simple and engaging.