Discover why enzymes work at body temperature while industrial catalysts need extreme heat – and what this means for the future of sustainable manufacturing
Quick Answer
“How does Chemical vs Biological Catalysis differ in terms of reaction conditions, efficiency, and selectivity?”
Chemical catalysts use synthetic materials and extreme conditions (high temperature/pressure) for industrial-scale reactions, while biological catalysts (enzymes) use proteins under mild conditions with superior selectivity and environmental benefits.
Chemical catalysts excel in harsh industrial environments, while biological catalysts dominate precision applications and sustainable manufacturing. The future lies in hybrid systems combining both approaches.
What You’ll Discover in This Complete Guide
✅ Why enzymes are 10 billion times faster than some chemical catalysts
✅ How pharmaceutical companies save 85% on waste using biological catalysts
✅ Which catalyst type consumes 2% of global energy (hint: it’s not enzymes)
✅ The $45 billion industry transforming manufacturing processes
✅ Real case studies from petroleum, pharma, and food industries
✅ Future hybrid technologies combining both catalyst types
✅ Cost breakdown showing why enzyme prices dropped 100x in recent years
✅ Environmental impact analysis revealing surprising sustainability winners
✅ Expert predictions for the next decade of catalytic innovations
✅ Practical decision framework for choosing the right catalyst type
Table of Contents
What Makes Catalysts So Revolutionary?
Ever wondered how your body breaks down a complex meal in hours, while factories need scorching temperatures and crushing pressures for similar reactions?
The secret lies in understanding chemical catalysis vs biological catalysis – two game-changing approaches that have revolutionized everything from life itself to trillion-dollar industries.
Here’s the mind-blowing reality: Catalysts make “impossible” reactions happen by providing molecular shortcuts that dramatically reduce the energy needed. But the way they achieve this couldn’t be more different.
Why This Matters Right Now
- $45 billion global catalyst market is reshaping industries
- Climate change urgency demands greener catalytic processes
- Pharmaceutical breakthroughs depend on precision catalysis
- Sustainable manufacturing requires understanding both approaches
Let’s dive deep into the seven critical differences that separate these catalytic powerhouses.
Chemical Catalysis Explained: The Industrial Workhorses
Chemical catalysis harnesses synthetic or inorganic substances to accelerate reactions through brute-force efficiency. Think platinum in your car’s catalytic converter or zeolites in oil refineries.
How Chemical Catalysts Dominate Industries
The Petroleum Industry Example:
- Catalytic cracking units operate at 500°C+
- Process millions of barrels daily
- Transform heavy oils into gasoline and diesel
- Generate $2+ trillion annually in refined products
Your Car’s Catalytic Converter:
- Uses platinum, palladium, and rhodium
- Converts toxic exhaust into harmless compounds
- Operates at 400-800°C
- Reduces emissions by 90%+
Two Main Types of Chemical Catalysts
- Homogeneous Catalysts
- Same phase as reactants
- Easier to study reaction mechanisms
- Harder to separate and reuse
- Heterogeneous Catalysts
- Different phase from reactants
- Easier separation and recycling
- More commonly used industrially
Key Insight: The $25 billion global chemical catalyst market proves these molecular workhorses are indispensable to modern manufacturing.
Biological Catalysis Decoded: Nature’s Precision Machines
Biological catalysis unleashes enzymes – sophisticated protein molecules evolved over millions of years to catalyze reactions with laser-like precision.
Why Enzymes Are Nature’s Masterpieces
Pepsin: Your Stomach’s Protein Shredder
- Functions at pH 1.5 (more acidic than lemon juice)
- Breaks down tough meat proteins effortlessly
- Operates at body temperature (37°C)
- Would destroy most synthetic catalysts
Catalase: The Speed Demon
- Decomposes hydrogen peroxide
- 200,000 reactions per second
- One of nature’s fastest catalysts
- Protects cells from oxidative damage
Beyond Natural Enzymes: Engineered Biological Catalysts
Modern biotechnology creates designer enzymes that:
- Perform reactions nature never intended
- Work in industrial conditions
- Achieve unprecedented selectivity
- Enable single-step pharmaceutical synthesis
Pharmaceutical Revolution Example: Statin production now uses engineered enzymes instead of 11-step chemical synthesis, reducing waste by 85%.
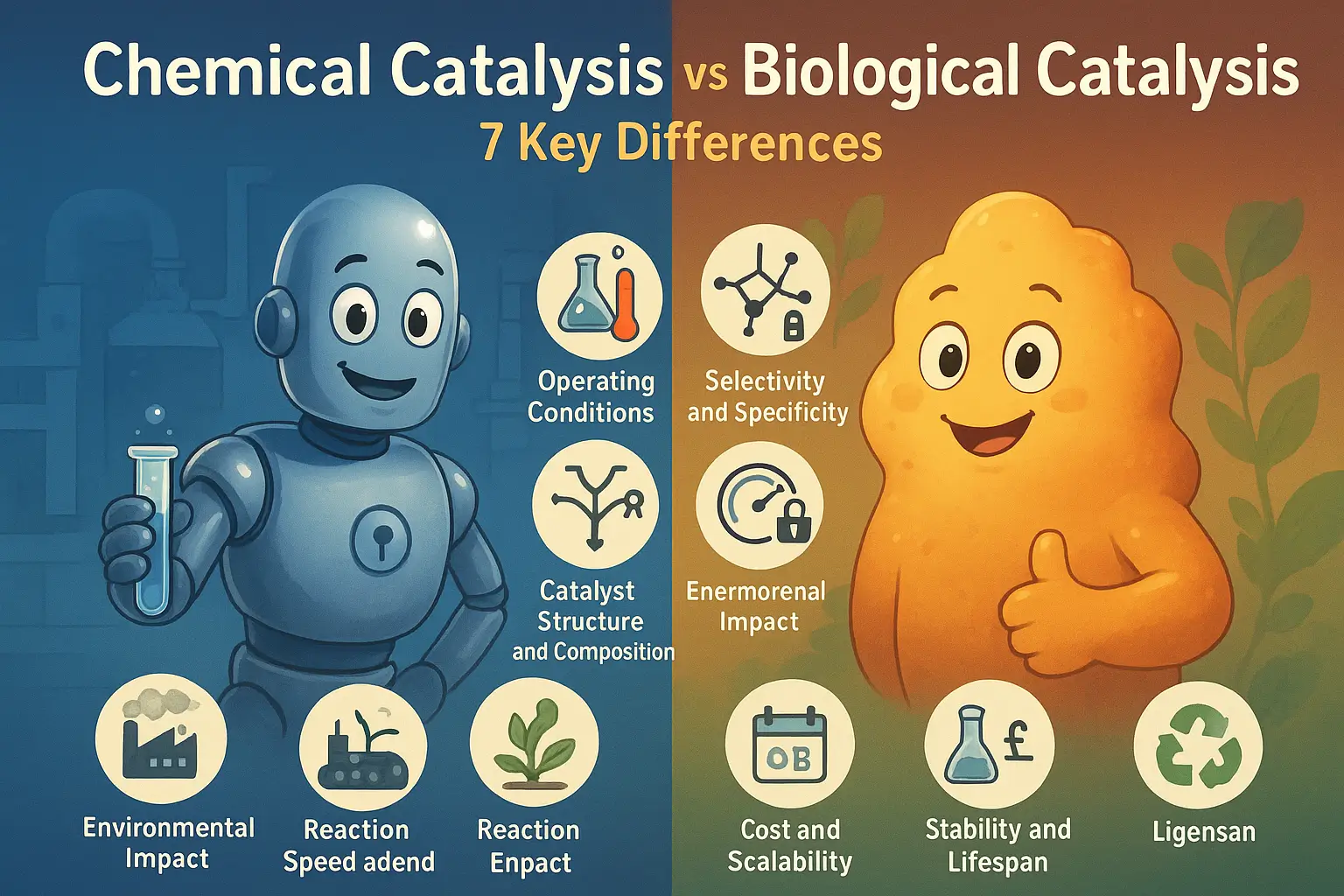
7 Critical Differences Between Chemical and Biological Catalysis
1. Operating Conditions: Extreme vs Gentle
| Aspect | Chemical Catalysis | Biological Catalysis |
|---|---|---|
| Temperature | 200-1000°C+ | 0-100°C (typically 37°C) |
| Pressure | Up to 200+ atmospheres | Atmospheric pressure |
| pH Range | 0-14 (extreme conditions) | 6-8 (near neutral) |
Real-World Impact:
Haber-Bosch Process (Chemical):
- Creates ammonia for fertilizers
- Requires 400-500°C and 200 atmospheres
- Consumes 2% of global energy
- Essential for feeding 40% of world’s population
Nitrogenase Enzyme (Biological):
- Also fixes nitrogen from air
- Works at room temperature
- Uses atmospheric pressure
- 10x more energy efficient
The Bottom Line: Biological catalysts achieve similar results with dramatically less energy consumption.
2. Selectivity: Shotgun vs Sniper Precision
Chemical Catalysts:
- Typically achieve 70-80% selectivity
- Generate unwanted by-products
- Require expensive purification
- Create industrial waste
Biological Catalysts:
- Achieve >99% selectivity regularly
- Produce single, desired products
- Eliminate purification costs
- Generate minimal waste
Pharmaceutical Game-Changer: Many drugs exist as mirror-image molecules where only one form works. Chemical synthesis produces 50:50 mixtures requiring separation. Enzymes produce 100% of the active form.
3. Structure: Simple vs Incredibly Complex
Chemical Catalysts:
- Simple structures (dozens of atoms)
- Easy to characterize and modify
- Straightforward manufacturing
- Predictable behavior
Biological Catalysts:
- Complex proteins (thousands of atoms)
- Intricate 3D architectures
- Refined through millions of years of evolution
- Multiple simultaneous functions
Analogy: Chemical catalysts are like efficient machines. Biological catalysts are like sophisticated robots with AI.
4. Speed: Fast vs Lightning Fast
Rate Enhancement Comparison:
- Chemical Catalysts: 1,000x to 1,000,000x faster than uncatalyzed reactions
- Biological Catalysts: 1,000,000x to 100,000,000,000,000,000x faster
The Champion: Orotidine monophosphate decarboxylase provides a 10¹⁷-fold rate enhancement – possibly the most efficient catalyst in the universe.
Why Enzymes Win: They achieve perfect substrate positioning AND transition state stabilization simultaneously.
5. Environmental Impact: Harsh vs Green
Chemical Catalysis Environmental Challenges:
- High energy consumption
- Toxic by-products
- Hazardous solvents
- Difficult disposal
Biological Catalysis Environmental Wins:
- Biodegradable proteins
- Aqueous reaction media
- Low energy requirements
- 30% reduction in carbon footprint (detergent industry example)
Sustainability Trend: Life cycle analyses increasingly favor biological processes for environmental performance.
6. Cost: Cheap vs Getting Cheaper
Chemical Catalysts:
- Low initial costs
- Established manufacturing
- Easy scalability
- Long operational lifespans
Biological Catalysts:
- Historically expensive
- Required specialized production
- Limited scalability
- But this is changing rapidly…
Revolution in Progress: Enzyme costs have dropped orders of magnitude in the past decade. Some industrial enzymes now cost less than $10 per kilogram.
7. Stability: Rock Solid vs Improving Rapidly
Chemical Catalysts:
- Withstand extreme conditions
- Maintain activity for months/years
- Resist poisoning and degradation
- Proven industrial reliability
Biological Catalysts:
- Traditionally temperature/pH sensitive
- Shorter operational lifespans
- But enzyme engineering is changing everything…
Modern Breakthrough: Engineered enzymes now maintain activity for weeks at elevated temperatures, rivaling chemical catalyst performance.
Which Catalyst Type Wins? Real-World Applications
The answer isn’t chemical OR biological – it’s chemical AND biological working together.
Pharmaceutical Industry: The Hybrid Approach
Simvastatin Success Story:
- Old Method: 11-step chemical synthesis
- New Method: Single enzymatic step
- Results: 99% selectivity, 85% waste reduction, eliminated heavy metals
Market Impact: Enzymatic pharmaceutical processes now represent a $7+ billion market.
Food & Beverage: Enzyme Revolution
High-Fructose Corn Syrup:
- Uses glucose isomerase enzyme
- Converts glucose to sweeter fructose
- Billion-dollar industry built on single enzyme
Brewing Industry:
- Multiple enzymes control fermentation
- Improve clarity and flavor
- Reduce processing time
Biofuels: Sustainable Energy Future
Cellulosic Ethanol:
- Enzymes convert agricultural waste to fuel
- Replaces fossil fuels sustainably
- Companies like Novozymes lead innovation
Textile Industry: Gentle Revolution
Enzyme Applications:
- Create stone-washed denim without stones
- Remove protein stains in detergents
- Replace harsh chemical treatments
- Reduce environmental impact by 50%+
The Future of Catalysis: What’s Coming Next?
1. Hybrid Catalytic Systems
Cascade Reactions combine chemical and biological catalysts in single vessels:
- Capture advantages of both approaches
- Minimize individual limitations
- Enable previously impossible transformations
2. AI-Powered Catalyst Design
Machine Learning Revolution:
- Predict enzyme structures and activities
- Accelerate catalyst development by 10x
- Design custom catalysts for specific reactions
3. Artificial Metalloenzymes
Best of Both Worlds:
- Protein selectivity + metal reactivity
- Completely new reaction capabilities
- Bridge chemical and biological approaches
4. Sustainable Manufacturing Integration
Flow Chemistry Advances:
- Continuous chemical processes
- Milder operating conditions
- Integration with biological systems
Next-Generation Bioreactors:
- Unprecedented scales for enzyme processes
- Enhanced efficiency and productivity
- Economic competitiveness with chemical processes
Quick Reference Comparison Table
| Aspect | Chemical Catalysis | Biological Catalysis | Winner |
|---|---|---|---|
| Operating Temp | 200-1000°C+ | 0-100°C | 🧬 Biological |
| Selectivity | 70-90% | >99% | 🧬 Biological |
| Rate Enhancement | 10³-10⁶ fold | 10⁶-10¹⁷ fold | 🧬 Biological |
| Environmental Impact | High waste/energy | Low waste/energy | 🧬 Biological |
| Industrial Scalability | Excellent | Improving rapidly | ⚗️ Chemical |
| Stability | Excellent | Improving rapidly | ⚗️ Chemical |
| Current Cost | Low | Decreasing rapidly | ⚗️ Chemical |
| Future Potential | Established | Revolutionary | 🧬 Biological |
Advantages and Disadvantages Comparison
Chemical Catalysis Advantages
Chemical catalysts excel in robust industrial environments where consistency and durability are paramount. Their ability to withstand extreme conditions makes them irreplaceable for many high-temperature, high-pressure processes.
The established infrastructure for chemical manufacturing provides immediate scalability advantages, whilst the relatively simple structures of chemical catalysts facilitate straightforward characterization and troubleshooting.
Cost-effectiveness for bulk production remains a significant advantage. Once optimized, chemical processes can produce enormous quantities of products with relatively low operating costs.
The long operational lifespans of chemical catalysts reduce replacement frequency and maintenance requirements.
Chemical Catalysis Disadvantages
Environmental concerns increasingly constrain chemical catalysis applications.
The energy-intensive nature of many chemical processes contributes significantly to carbon emissions, whilst the generation of toxic by-products raises disposal and safety concerns.
Limited selectivity often necessitates complex separation processes, increasing costs and waste generation.
The inflexibility of chemical catalysts can also be problematic. Modifying reaction conditions or switching to alternative products often require entirely new catalyst systems, limiting process adaptability.
Biological Catalysis Advantages
Environmental sustainability represents the primary advantage of biological catalysis. The biodegradable nature of enzymes, combined with mild operating conditions and aqueous reaction media, creates inherently green processes.
The extraordinary selectivity of biological catalysts eliminates waste generation whilst producing single, desired products.
The programmability of biological systems offers unprecedented flexibility. Enzymes can be engineered to catalyze non-natural reactions, whilst directed evolution enables the development of catalysts with precisely defined properties. This adaptability allows biological systems to tackle previously impossible synthetic challenges.
Biological Catalysis Disadvantages
Sensitivity to operating conditions remains a significant limitation, though enzyme engineering continues to address this challenge.
The complexity of biological systems can make troubleshooting difficult, particularly when multiple enzymes are involved in cascade reactions.
Traditional cost disadvantages are diminishing but haven’t disappeared entirely.
High-value applications justify enzymatic processes, but bulk commodity production may still favor chemical approaches for purely economic reasons.
Frequently Asked Questions
Q: Which catalyst type is better for the environment?
A: Biological catalysts are generally more environmentally friendly due to:
Biodegradable nature (break down into harmless amino acids)
Lower energy requirements (operate at room temperature)
Minimal toxic by-products
Aqueous reaction conditions (no harmful solvents)
Q: Why don’t we use biological catalysts for everything?
A: While biological catalysts offer many advantages, chemical catalysts are still essential for:
High-temperature processes (>100°C)
Extreme pH conditions
Large-scale commodity production
Processes requiring long catalyst lifespans
Q: How much do industrial enzymes cost?
A: Enzyme costs have plummeted dramatically:
2000s: $1,000-10,000 per kg
2025: $10-1,000 per kg for many industrial enzymes
Future: Continued cost reductions expected through improved production
Q: Can enzymes be engineered for non-natural reactions?
A: Absolutely! Modern protein engineering enables:
Directed evolution for enhanced properties
Completely artificial reactions
Custom enzymes for specific industrial needs
Combination of natural and synthetic features
Q: What industries are switching to biological catalysts?
A: Major transitions occurring in:
Pharmaceuticals (chiral drug synthesis)
Detergents (stain removal, fabric care)
Food processing (sweetener production, brewing)
Biofuels (cellulose conversion)
Textiles (fabric treatment, dyeing)
Conclusion
The battle between chemical catalysis vs biological catalysis isn’t about choosing sides – it’s about understanding when to deploy each molecular weapon in your arsenal.
Key Takeaways:
- Chemical catalysts excel in harsh industrial environments requiring robustness and massive scale
- Biological catalysts dominate when precision, sustainability, and energy efficiency matter most
- Hybrid systems combining both approaches represent the most exciting frontier
- Cost barriers for biological catalysts are disappearing rapidly
- Environmental pressures increasingly favor biological solutions
What This Means for You:
If you’re a researcher or engineer: Consider biological alternatives for new projects, especially those requiring high selectivity or operating under environmental constraints.
If you’re a business leader: Evaluate enzyme-based processes for competitive advantages in sustainability and product quality.
If you’re a student: Master both catalytic approaches – the future belongs to those who can integrate chemical and biological systems creatively.
The catalytic revolution is accelerating. Whether you’re developing life-saving drugs, creating sustainable fuels, or manufacturing everyday products, understanding these seven critical differences will help you choose the right catalytic strategy for maximum impact.